To determine risk factors during neonatal hospital stay and follow-up associated with failure to thrive in the first year of life of very low birth weight newborns.
MethodsStudy of preterm very low birth weight newborns followed from 2006 to 2013 in a public institutional hospital program. The study included newborns that attended at least one appointment in each of the three periods: Period I, up to 3 months of corrected age (CA); Period II, 4–6 months of CA; and Period III, 7–12 months of CA. The variables were analyzed by logistic regression with XLSTAT 2014 software (Microsoft®, WA, USA). Failure to thrive (Z-score below −2 SD) was classified as a dichotomous dependent variable (0 – failure/1 – success), while the other variables were classified as explanatory variables for the hospitalization periods and for each of the follow-up periods (I, II, and III).
ResultsChildren born adequate for gestational age increased the chance of Z-score for weight at discharge>−2 SD (OR=10.217; 95% CI: 1.117–93.436). Metabolic bone disease and retinopathy of prematurity in Period I, as well as hospital readmissions in Periods II and III during follow-up increased the chance of Z-score<−2 SD.
ConclusionFailure to thrive is influenced by intrauterine factors and, subsequently, by several morbidities, both in the birth and hospitalization period, as well as in the post-discharge period and thus, such variables should be prioritized in the follow-up.
Determinar fatores de risco do período de internação neonatal e do seguimento ambulatorial associados à falha de crescimento no primeiro ano de vida de recém-nascidos de muito baixo peso.
MétodosEstudo com crianças nascidas prematuras de muito baixo peso em acompanhamento de 2006 a 2013 em Ambulatório de Alto Risco de um Hospital Escola. Incluídas aquelas que realizaram pelo uma consulta em cada um dos três períodos assim determinados: Período I – até 3 meses de Idade Corrigida (IC); Período II – entre 4 a 6 meses de IC e Período III – entre 7 a 12 meses de IC. As variáveis foram analisadas por regressão logística com programa XLStat 2014 (Microsoft®, WA, EUA). A falha de crescimento (escore z abaixo de −2 DP) classificada como variável dependente do tipo dicotômica (0 – falha/1 – sucesso) e demais variáveis classificadas como variáveis explicativas para os períodos de internação e para cada um dos períodos de seguimento (I, II e III).
ResultadosNascer Adequado para a Idade Gestacional aumenta a chance de apresentar escore Z do peso na alta hospitalar acima de −2 DP (OR=10,217; IC95% 1117–93,436). Doença Metabólica Óssea e Retinopatia da Prematuridade, durante o Período I e reinternações nos Períodos II e III de seguimento aumentam a chance de escore Z abaixo de −2 DP.
ConclusãoA falha de crescimento é influenciada por fatores intrauterinos e posteriormente por diversas morbidades, tanto no período da internação como no pós-alta, tais variáveis estudadas deveriam ser priorizadas no seguimento.
Failure to thrive during early childhood can have permanent harmful effects, especially in preterm infants (PI)1 as growth, mainly in those born with very low birth weight (VLBW), is influenced by intrauterine and birth factors, as well as variables during hospitalization and post-hospital discharge,2 causing future problems such as neurodevelopmental alterations3,4 and metabolic syndrome.5,6
Studies have addressed the influence of the hospitalization period and the first years of life after discharge on the growth of PI,7–10 demonstrating that birth (weight, weight/GA ratio) and hospitalization (hospital length of stay, presence of hyaline membrane disease) variables have an effect on growth in the short- and long term.11
During hospitalization, the VLBW preterm infant has restricted growth, with significantly lower rates than the intrauterine rates. Most of these PI are born weighing between the 10th and 90th percentiles of the intrauterine growth curve, considered adequate for gestational age – (AGA). However, at the discharge from the neonatal intensive care unit (NICU) or at 36 weeks post-conception age, they are below the 10th percentile of the same curve, characterizing extrauterine growth restriction (EUGR).
This situation influences the prognosis of PI, both in relation to growth and to development, leading to failure to thrive in childhood, stunting, and underweight, with consequences in adulthood.3,12 Factors associated with EUGR include: nutritional practice, male gender, need for ventilation on the first day of life, use of mechanical ventilation for long periods, hospital length of stay, and complications inherent to premature birth, such as bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), and late sepsis.13
The post-hospital discharge and outpatient follow-up periods can also be accompanied by several complications, such as BPD, which favors frequent respiratory complications, resulting in recurrent hospitalizations early in life; gastroesophageal reflux; presence of visual and auditory deficits; psychomotor developmental delays; and cerebral palsy.14,15
Given the current survival rates of PI, especially those with VLBW, it becomes prudent to search for better long-term results, with growth constituting the critical point to be emphasized in the care of premature infants. Thus, the authors emphasize the need to identify complications during the hospital stay and post-discharge periods to understand the growth dynamics of the PI discharged from the NICU. Hence, the aim of this study was to assess variables during the NICU hospitalization and outpatient follow-up periods that can influence failure to thrive in VLBW PI.
MethodsThis study was carried out based on medical records of preterm children born with VLBW, followed at the High-Risk Outpatient Clinic of a teaching hospital located in the Western region of the state of Paraná, Brazil. This service treated 305 children from the NICU during the study period; of this total, 101 were VLBW PI, the subject of the present study.
The study included the VLBW PI followed between 2006 and 2013 that had, after hospital discharge, at least three appointments at the High-Risk Outpatient Clinic during the first 12 months of life, with at least one consultation in each period of the study, as follows: Period I, up to 3 months corrected age (CA); period II, between 4 and 6 months CA; and period III, between 7 and 12 months CA. The CA was considered as the chronological age minus the weeks of gestational age at birth, subtracted from 40 weeks.
Patients with severe congenital malformations, those not hospitalized at birth in the NICU of the research hospital, or those who died during follow-up were excluded. Of the total of 101 VLBW PI, there was a loss of 30 patients, of which 22 (73%) had fewer appointments or at different periods than those determined by the study to assess the PI. Therefore, the study sample comprised 71 VLBW PI, with the power of analysis of 0.84 calculated by G Power software version 3.1, available at: http://www.gpower.hhu.de/en.html, considering in the logistic regression an error type 1 of 0.1; error type 2 of 0.2, and significant relative risk of 2.
To correlate weight/GA and calculate the Z-score of the anthropometric variables weight, height, and head circumference (HC) at birth and at the time of hospital discharge, the Fenton and Kim curve1 was used with the help of Fenton Growth Chart Calculator, available at: http://www.ucalgary.ca/fenton/. The Z-score of the anthropometric variables of the follow-up period was calculated using the anthropometric calculator of the Anthro program (2011), available at: http://www.who.int/childgrowth/software/en/. When there were more appointments during the assessed periods, the Z-score was calculated for each appointment and then the average was obtained for each follow-up period.
Data were entered into Microsoft Excel® 2010 (Microsoft®, WA, USA), using the CA, and descriptive statistics were performed (minimum, maximum, mean, standard deviation, relative frequency). The variables were analyzed regarding the distribution pattern using the Shapiro–Wilks test, followed by the homogeneity test of variance through the F-test. The variables that were in accordance with the assumptions of normality and homoscedasticity were analyzed between groups children that met the inclusion criteria with those who were not in accordance with the criteria (losses), using Student's t-test for independent samples. Other variables that were not in accordance with the statistical assumptions were assessed using the nonparametric Mann–Whitney U-test.
The variables were then analyzed by logistic regression. Failure to thrive (Z-score<−2) was classified as a dichotomous dependent variable (0 – failure/1 – success) and the other variables were classified as explanatory variables for the periods of hospitalization and for each of the follow-up periods (I, II, and III). The explanatory variables of the hospitalization period were: gender, weight/GA classification; time of birth weight recovery; percentage of weight lost during hospitalization; hospital length of stay.
For the follow-up period, the explanatory variables were: gastroesophageal reflux (GER) – (considered as the presence of abundant and frequent vomiting after feedings); retinopathy of prematurity (ROP – considering stages 3, 4, and 5); BPD (defined as the use of oxygen at 28 days of life); use of oxygen at hospital discharge; metabolic bone disease (MBD) – (regarded as alkaline phosphatase serial measurements>900mg/dL, calcium, and phosphorus associated with clinical and radiological criteria), and rehospitalization during the post-discharge follow-up period.
Therefore, models were created for the anthropometric parameters for the time of discharge, as well as one model for each follow-up period after discharge from the NICU. The explanatory admission and postnatal variables were applied to each model separately. The logit model was used for the purpose of logistic regression analysis, using the stepwise-forward method with binary response. The receiver operating characteristic (ROC) curve was adjusted using the Hosmer and Lemeshow model. At the end of the adjustment, the sensitivity (the proportion of true positives) and specificity (the proportion of true negatives) were calculated, as well as the area under the ROC curve that represents the model adjustment explicability, showing how the model discriminates the outcome (growth). All statistical analyses were performed using XLStat software, 2014 version, available at: https://www.xlstat.com/en/.
The study was approved by the Ethics Committee on Human Research of Universidade Estadual do Oeste do Paraná (UNIOESTE), opinion No. 385.407.
ResultsA total of 71 VLBW PI was evaluated, of which 36 were males, with most children born by cesarean section (59%) and mean GA of 29.4±2.8 weeks, with 70% of children classified as AGA. At discharge, 68 children (95.8%) were below the 10th percentile of the Fenton and Kim curve.1 At admission, 43 (61%) used parenteral nutrition (PN), for a mean of 21.38±13.90 days. The percentage of lost birth weight averaged 13.64±6.18%. This weight loss occurred within 5.27±2.60 days and the VLBW PI took an average of 14.96±5.82 days to recover the birth weight. The length of stay was 68.73±27.26 days. At hospital discharge, mean Z-scores for weight, length, and HC were: −3.05±1.21; −2.23±1.14, and −1.5±1.45.
The 30 VLBW PI not included in the study, as they did not meet the established criteria, had mean GA of 29.33±2.77 weeks (p=0.895); 23 (73%) were AGA (p=0.541), mean birth weight 1154.16±274.08g (p=0.167); had length of 36.83±2.47cm (p=0.557), HC of 27±1.62cm (p=0.288), and birthweight Z-score of −1.0±0.9 (p=0.074). Significantly similar values were observed when comparing the excluded children with those included in the study.
For the 71 VLBW PI, mean Z-scores of the follow-up periods I, II, and III of each anthropometric variable showed that weight ranged from −2.4±1.3 to −1.2±1.3, length varied from −2.5±1.5 to −1.1±1.4, and HC ranged from −1.1±1.6 to −0.5±1.5 between the first and third periods.
At birth, 12 (17%) VLBW PI were below −2 SD for weight, 29% for height and 13% for HC. At discharge, 57 (80%) were below −2 SD for weight. During the follow-up from Period I until Period III, there was a decrease in the percentage of PI below the Z-scores<−2 SD; regarding weight, it decreased from 49% to 27%, height from 61% to 25%, and HC from 22% to 14% in Period III.
The most common morbidities in the follow-up period were GER, ROP, and BPD. MBD was observed in Periods I and II of clinical follow-up. In Period I, 13 (18.3%) children were rehospitalized, as were 12 (17%) in Periods II and III. The main cause of rehospitalization was respiratory problems, with pneumonia being the most common.
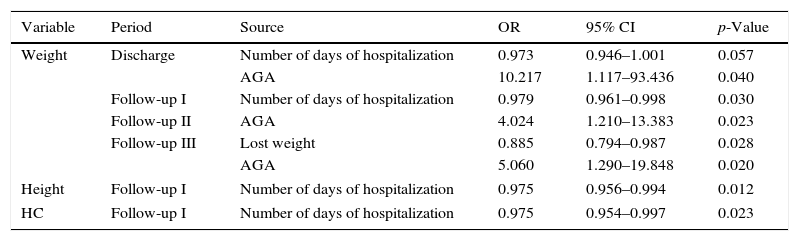
When evaluating the predictive models, it was observed that the fact of being born AGA made children 10.3 times more likely to have a weight Z-score at discharge>−2 (OR=10.217, 95% CI: 1.117–93.436; p=0.04), in addition to increasing by 4.024- and 5.060-fold the chance of the weight Z-score>−2 during Periods II (OR=4.024, 95% CI: 1.210–13.383, p=0.023) and III (OR=5.060, 95% CI: 1.290–19.848; p=0.020) of the outpatient follow-up, respectively (Table 1).
Hospital admission variablesa and growth failure in the follow-up of very low birth weight preterm infants.
| Variable | Period | Source | OR | 95% CI | p-Value |
|---|---|---|---|---|---|
| Weight | Discharge | Number of days of hospitalization | 0.973 | 0.946–1.001 | 0.057 |
| AGA | 10.217 | 1.117–93.436 | 0.040 | ||
| Follow-up I | Number of days of hospitalization | 0.979 | 0.961–0.998 | 0.030 | |
| Follow-up II | AGA | 4.024 | 1.210–13.383 | 0.023 | |
| Follow-up III | Lost weight | 0.885 | 0.794–0.987 | 0.028 | |
| AGA | 5.060 | 1.290–19.848 | 0.020 | ||
| Height | Follow-up I | Number of days of hospitalization | 0.975 | 0.956–0.994 | 0.012 |
| HC | Follow-up I | Number of days of hospitalization | 0.975 | 0.954–0.997 | 0.023 |
OR, odds ratio; 95% CI, 95% confidence interval; Period I, up to 3 months corrected age; Period II, 4–6 months corrected age; Period III, 7–12 months corrected age; AGA, adequate for gestational age.
Longer hospital length of stay of the VLBW PI was associated with a 1.027-fold increased chance of weight at discharge<−2 SD (OR=0.973, 95% CI 0.946–1.001; p=0.057). A similar result was observed for the Z-scores of weight, height, and HC in the outpatient period. Higher percentage of weight lost during hospitalization in the NICU was associated with a 1.129-fold higher chance to fail at weight gain in Period III of follow-up (OR=0.885, 95% CI 0.794–0.987; p=0.028; Table 1).
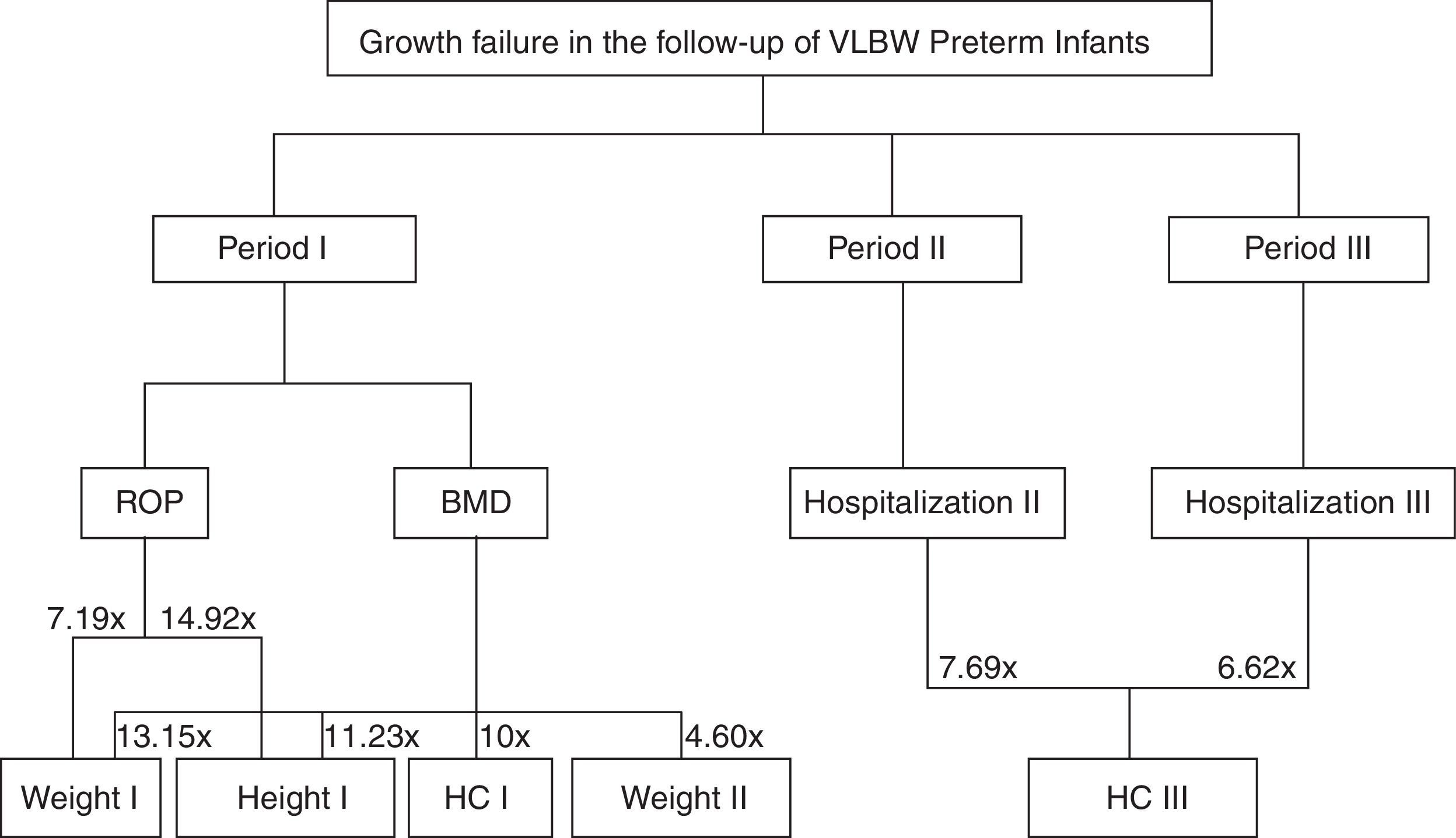
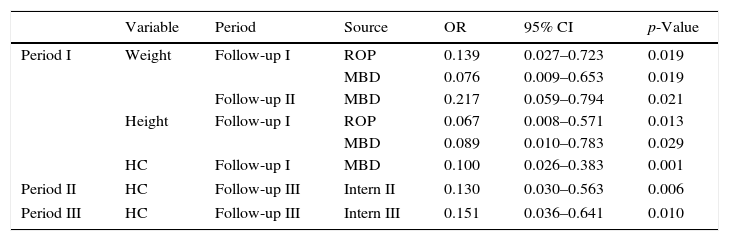
The presence of MBD in Period I increased by more than 10-fold the risk to obtain a score<−2 SD in the same period for all assessed anthropometric parameters. Also, during Period II, there was a 4.608 higher chance of weight being<−2 SD.
The occurrence of ROP in Period I increased by 7.194-fold the risk of failure to thrive for weight (OR=0.139; 95% CI: 0.027–0.723; p=0.019) and by 14.925-fold for height (OR=0.067, 95% CI: 0.008–0.571; p=0.013) (Table 2).
Outpatient follow-up variablesa and growth failure in the follow-up of very low birth weight preterm infants.
| Variable | Period | Source | OR | 95% CI | p-Value | |
|---|---|---|---|---|---|---|
| Period I | Weight | Follow-up I | ROP | 0.139 | 0.027–0.723 | 0.019 |
| MBD | 0.076 | 0.009–0.653 | 0.019 | |||
| Follow-up II | MBD | 0.217 | 0.059–0.794 | 0.021 | ||
| Height | Follow-up I | ROP | 0.067 | 0.008–0.571 | 0.013 | |
| MBD | 0.089 | 0.010–0.783 | 0.029 | |||
| HC | Follow-up I | MBD | 0.100 | 0.026–0.383 | 0.001 | |
| Period II | HC | Follow-up III | Intern II | 0.130 | 0.030–0.563 | 0.006 |
| Period III | HC | Follow-up III | Intern III | 0.151 | 0.036–0.641 | 0.010 |
OR, odds ratio; 95% CI, 95% confidence interval; Period I, up to 3 months corrected age; Period II, 4–6 months corrected age; Period III, 7–12 months corrected age; MBD, metabolic bone disease; ROP, retinopathy of prematurity; GER, gastroesophageal reflux.
Re-hospitalization in Period II increased by 7.692-fold the chance of HC being<−2 SD in Period III (OR=0.130, 95% CI: 0.030–0.563; p=0.006). Likewise, rehospitalization in the Period III increased by 6.622-fold the risk of Z-scores<−2 SD for HC (OR=0.151, 95%CI: 0.036–0.641; p=0.010).
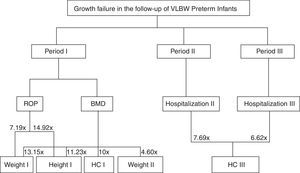
Fig. 1 shows a flow chart from Table 2, demonstrating the complications for each follow-up period and failures in their respective anthropometric variables.
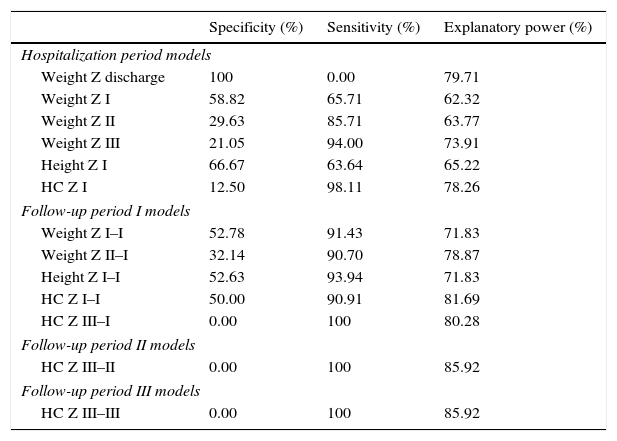
Based on the ROC curve, the specificity, sensitivity, and explanatory power of the variables of hospital stay and the three follow-up periods were determined. The weight at discharge appears to be a good predictor among the variables that constitute the model of neonatal hospitalization, whereas in the three periods of outpatient follow-up, Z-score variations of head circumference (HC) showed better explanatory power (Table 3).
Specificity, sensitivity, and explanatory power of variables during the hospital stay and follow-up periods.
| Specificity (%) | Sensitivity (%) | Explanatory power (%) | |
|---|---|---|---|
| Hospitalization period models | |||
| Weight Z discharge | 100 | 0.00 | 79.71 |
| Weight Z I | 58.82 | 65.71 | 62.32 |
| Weight Z II | 29.63 | 85.71 | 63.77 |
| Weight Z III | 21.05 | 94.00 | 73.91 |
| Height Z I | 66.67 | 63.64 | 65.22 |
| HC Z I | 12.50 | 98.11 | 78.26 |
| Follow-up period I models | |||
| Weight Z I–I | 52.78 | 91.43 | 71.83 |
| Weight Z II–I | 32.14 | 90.70 | 78.87 |
| Height Z I–I | 52.63 | 93.94 | 71.83 |
| HC Z I–I | 50.00 | 90.91 | 81.69 |
| HC Z III–I | 0.00 | 100 | 80.28 |
| Follow-up period II models | |||
| HC Z III–II | 0.00 | 100 | 85.92 |
| Follow-up period III models | |||
| HC Z III–III | 0.00 | 100 | 85.92 |
Period I, up to 3 months corrected age; Period II, 4–6 months corrected age; Period III, 7–12 months corrected age.
The main variable that influenced failure to thrive in VLBW PI during hospital stay and over the 12 months of CA was SGA, while those born AGA had 10.3 times more chance of having a Z-score for weight at discharge>−2 SD. The presence of MBD and ROP predicts risk for failure to thrive in the first and second trimesters of life of VLBW PI. Additionally, rehospitalization in the second and third trimesters of CA increased the chances of failure to thrive for the HC by 7.7- and 6.6-fold, respectively.
EUGR is always considered to be a risk situation in the NICU, with variable frequencies in studies carried out in several countries: 63% in India,14 57% in Norway,16 63.5% in the state of Rio de Janeiro, Brazil,7 and 39.1% in a study conducted in the same city, but approximately a decade later.17 Among the assessed preterm infants, it was found that 95.6% were below the 10th percentile, a much higher EUGR rate than those found in the abovementioned studies. Of the PI that had EUGR, 12.6% had height<−2 SD in childhood, which was more common in the male gender.18
Although the Z-scores of birth weight were similar8 at the time of hospital discharge, there was a significant reduction when compared to the Z-score at discharge in the same study carried out in Southeastern Brazil (3.05 vs. −1.79). The present study includes children born before 2010, a period when there were different routines regarding nutrition. Standardized care practices and routines of aggressive PN and early enteral nutrition are necessary to try to avoid or minimize EUGR, which may reduce by up to 2.17-fold the risk of Z-score≤−2 SD.7 Supplementation with choline, Uridine, and docosahexaenoic acid is being investigated aiming to improve growth in high-risk newborns.19 Additionally, the fact that there is a multidisciplinary team in parenteral nutrition for nutritional management reduces the incidence of EUGR from 62.6% to 44%.20
Being born SGA and have inadequate growth in the first year of life are risk factors for growth alterations at 24 months of CA.21 Still, similar to other studies, in which being born SGA increased by 12.19 times the Z-score≤−2 SD7 at term and increased by 3.41 times the risk of Z-score≤−2 SD at discharge,17 being born SGA also represented an additional risk for EUGR.
The failure to thrive, i.e., Z-score decrease>0.67 in follow-up periods, occurred in 28% of VLBW PI in Period II (between 4 and 8 months CA),22 with BPD representing a significant predictor of failure to thrive for the Periods I (40 weeks to 4 months CA) and II of follow-up. The present study found no significant results for growth and presence of BPD; the morbidities occurring during outpatient follow-up that showed an influence on failure to thrive were MBD and the presence of ROP. Similar results showed there was no association between failure to thrive and chronic pulmonary disease; however, it was strongly influenced by severe ROP.23
In a study22 with extremely low birth weight PI, followed until 20 months of CA, the hospitalization rate was 40% during the entire follow-up period, with no statistical difference between those who showed failure to thrive (n=62; 40%) and those who did not (n=92; 60%). The present study's rehospitalization rate was higher (52%) and the occurrence of hospitalization during Periods II and III increased the chance of failure to thrive for the HC in Period III. This aspect is particularly relevant because in this phase of life, appropriate HC growth is essential for normal development.24
Differences in the growth patterns after the discharge of VLBW PI should be considered. The findings shown here allow us to verify progressive increase in the value of the Z-score for weight, height, and HC throughout the outpatient follow-up. Although at 12 months of CA the obtained rates of these anthropometric parameters were lower than those found in Southern Brazil,10 the growth rates were higher than those in an Indian study.14 Thus, it is observed that one should consider, in addition to neonatal and follow-up complications inherent to preterm birth, the environment where the child lives, as well as socioeconomic, nutritional, educational, and regional cultural factors.2 Many models of anthropometric variables considered significant, defined in the neonatal period and Periods I, II, and III of follow-up, showed high sensitivity and intermediate specificity, i.e. they define high probability of detecting cases of growth success, and moderately the probability of detecting cases of failure to thrive. HC showed high sensitivity, but zero specificity, except for the neonatal period. The definition of three periods for critical evaluation of the PI growth, allows a closer look by the professional to detect early failures and promote appropriate growth patterns during those moments.
Based on these findings, it can be concluded that the main factor that influenced the VLBW PI throughout the hospital stay was SGA, which during hospital discharge and also over the 12 months of CA, increased failure to thrive. The presence of MBD and ROP significantly influence growth in the first quarter of the first 12 months of life of VLBW PI.
Although growth is also influenced by maternal and family socioeconomic factors,25 it was not possible to verify this influence in the present study, as it was retrospective, in addition to the fact that this information was not included in the reviewed medical records, significantly limiting the findings. Similarly, the number of PI that were excluded for not having the minimum number of three appointments during the first year of life constituted a limiting factor to extend the results of this study. The difficulty to perform long-term follow-up is also found in other contexts.26,27
The growth of VLBW PI during the first 12 months of life is influenced by several factors, from the nutritional status in utero, nutritional practices in the NICU and the follow-up period, as well as regional, cultural, and environmental aspects, which need to be clarified in further studies designed for this purpose.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Rover MM, Viera CS, Silveira RC, Guimarães AT, Grassiolli S. Risk factors associated with growth failure in the follow-up of very low birth weight newborns. J Pediatr (Rio J). 2016;92:307–13.