Mannose-binding lectin, which belongs to the collectin family, is an acute-phase reactant that activates the complement system. This study aimed to investigate the effect of MBL2 gene polymorphism on short-term outcomes in preterm infants.
MethodInfants of <37 gestational weeks who were admitted to the neonatal intensive care unit during a two-year period were enrolled in this prospective study. The neonates were categorized into two groups according to their MBL2 genotypes. Normal MBL2 genotype was defined as MBL2 wild-type (AA genotype), whereas mutant MBL2 genotype was defined as MBL2 variant-type (AO/OO genotype). The relationship between MBL2 genotype and short-term morbidity and mortality was evaluated.
ResultsDuring the two-year study period, 116 preterm infants were enrolled in this study. In MBL2 variant-type, mannose-binding lectin levels were significantly lower and incidences of mannose-binding lectin deficiency (MBL level<700ng/mL) were higher (p<0.001). In this group, the prevalence of respiratory distress syndrome and mortality was significantly higher (p<0.001, p=0.03 respectively). In the MBL2 wild-type group, the prevalence of necrotizing enterocolitis (NEC) was higher (p=0.01). Logistic regression analyses revealed that MBL2 variant-type had a significant effect on respiratory distress syndrome development (odds ratio, 5.1; 95% confidence interval, 2.2–11.9; p<0.001).
ConclusionsMBL2 variant-type and mannose-binding lectin deficiency are important risk factors for respiratory distress syndrome development in preterm infants. Additionally, there is an association between MBL2 wild-type and NEC. Further studies on this subject are needed.
A lectina ligante de manose (MBL, do inglês mannose-binding lectin), que pertence à família das colectinas, é um reagente de fase aguda que ativa o sistema complemento. Este estudo teve como objetivo investigar o efeito do polimorfismo do gene MBL2 em desfechos de curto prazo em prematuros.
MétodoEste estudo prospectivo incluiu crianças com menos de 37 semanas de gestação admitidas na unidade de terapia intensiva neonatal durante dois anos. Os neonatos foram categorizados em dois grupos de acordo com os genótipos do MBL2. O genótipo normal do gene MBL2 foi definido como MBL2 do tipo selvagem (genótipo AA), enquanto o genótipo mutante do gene MBL2 foi definido como o gene variante (genótipo AO/OO). Foi avaliada a relação entre o genótipo MBL2 e a morbidade e mortalidade em curto prazo.
ResultadosDurante o período de dois anos, 116 bebês prematuros foram incluídos neste estudo. Os níveis de lectina ligante de manose foram significativamente menores nos variantes do MBL2 e as incidências de deficiência de lectina ligante de manose (nível de MBL < 700 ng/mL) foram maiores (p < 0,001). Nesse grupo, a prevalência de síndrome do desconforto respiratório (SDR) e a mortalidade foram significativamente maiores (p < 0,001, p = 0,03, respectivamente). No grupo MBL2 do tipo selvagem, a prevalência de enterocolite necrosante foi maior (p = 0,01). Análises de regressão logística revelaram que os genes variantes do MBL2 apresentaram um efeito significativo no desenvolvimento da síndrome do desconforto respiratório (odds ratio, 5,1; intervalo de confiança de 95%, 2,2–11,9; p < 0,001).
ConclusõesAs variantes do MBL2 e a deficiência de lectina ligante de manose são importantes fatores de risco para o desenvolvimento da síndrome do desconforto respiratório em neonatos prematuros. Além disso, existe uma associação entre MBL2 do tipo selvagem e a enterocolite necrosante. Mais estudos são necessários sobre esse assunto.
Mannose-binding lectin (MBL) is an acute-phase reactant that activates the complement system. It belongs to the collectin family of proteins, which includes lung surfactant protein A (SP-A) and SP-D.1 MBL plays a key role in first-line immune responses as a component of innate immunity.2 Because adaptive immunity is underdeveloped in preterm infants, innate immunity gains higher importance.2,3 The MBL2 gene is located on the long arm of chromosome 10, and mutant MBL2 alleles occur as a result of three single-point mutations in this gene (B, C, and D). Although functional MBL levels are low in heterozygous polymorphisms, MBL levels in homozygous polymorphisms are so low that they may not even be determinable.4,5 MBL activates the complement system by binding to mannose or sugar motifs, which are found in many microorganisms, and plays an important role in innate immunity and inflammation.2,6 In newborns, an increase in sepsis frequency is observed when MBL levels are low.2,3,7
The mortality and morbidity rates in preterm infants are higher than those in term infants. As gestational week and birth weight decrease, the risk of complications increases. In preterm infants, complications are observed in the early (neonatal period) and late periods (after discharge). Although the survival rate of most preterm infants has improved because of advances in medical care, the incidence of short-term complications remains relatively stable. Short-term complications increase the risk of long-term sequelae.8,9
In recent years, many studies have been conducted on the importance of MBL during the neonatal period, and most of these are associated with sepsis. In sepsis, proinflammatory and anti-inflammatory cytokine ratio is vital in terms of defense against infectious agents. The imbalance in this ratio is manifested by increased morbidity and mortality during the neonatal period.5,6 Increased cytokine levels have a significant role in the pathophysiology of morbidities such as respiratory distress syndrome (RDS), intraventricular hemorrhage (IVH), necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), and retinopathy of prematurity (ROP).10 This prospective study aimed to investigate the association of MBL2 polymorphism with short-term outcomes in preterm infants.
Materials and methodsAll preterm infants of <37 gestational weeks who were admitted to the neonatal intensive care unit (NICU) of the Uludag University Medical School during a two-year period were enrolled in this prospective study. The neonates were categorized into two groups according to their MBL2 genotypes. Normal MBL2 genotype was defined as MBL2 wild-type (AA genotype), whereas mutant MBL2 genotype was defined as MBL2 variant-type (AO or OO genotype). The exclusion criteria included refusal of parental consent, infants with major congenital abnormalities, and those undergoing a major surgical procedure.
Gestational age, birth weight, gender, mode of delivery, Apgar score at 1 and 5min, prenatal demographics, antenatal steroid administration, premature rupture of membranes, history of chorioamnionitis and durations of invasive mechanical ventilation, total supplemental oxygen, central catheterization, and total parenteral nutrition were recorded. The presence of neonatal morbidities such as RDS, late-onset sepsis (LOS), IVH, NEC, BPD, ROP, and the mortality data of the preterm infants were recorded.
RDS was diagnosed based on clinical findings (tachypnea, retractions, nasal flaring, and cyanosis) or radiological findings (reticular granular pattern or air bronchograms). All neonates underwent the same management according to the NICU protocols and as recommended by European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants.11,12 Neonatal sepsis was defined as the presence of clinical signs of sepsis with a positive blood culture. Blood cultures were analyzed using the fully automated BACTEC method in a BACTEC 9240 device (Becton Dickinson, Heidelberg, Germany). LOS was determined by the time at which sepsis occurred between 4 and 30 days after birth.13 IVH was evaluated by cranial ultrasound examinations, which were performed by the same pediatric radiologist and diagnosed using the Papile classification system.14 NEC was diagnosed according to clinical and radiographic findings and classified according to modified Bell's criteria.15 BPD was classified into three groups in terms of BPD severity depending on the duration and level of supplemental oxygen and mechanical ventilatory support at 36 weeks postmenstrual age.16 ROP was classified according to the International Classification of Retinopathy of Prematurity.17
The MBL levels and gene polymorphisms were assessed within 3h in most infants and within 24h after birth in all infants. The blood samples for the measurement of MBL levels were collected in a test tube and these blood samples were centrifuged within thirty minutes after obtaining. After the centrifugation process, serum of the samples were immediately stored at −80°C until analysis.
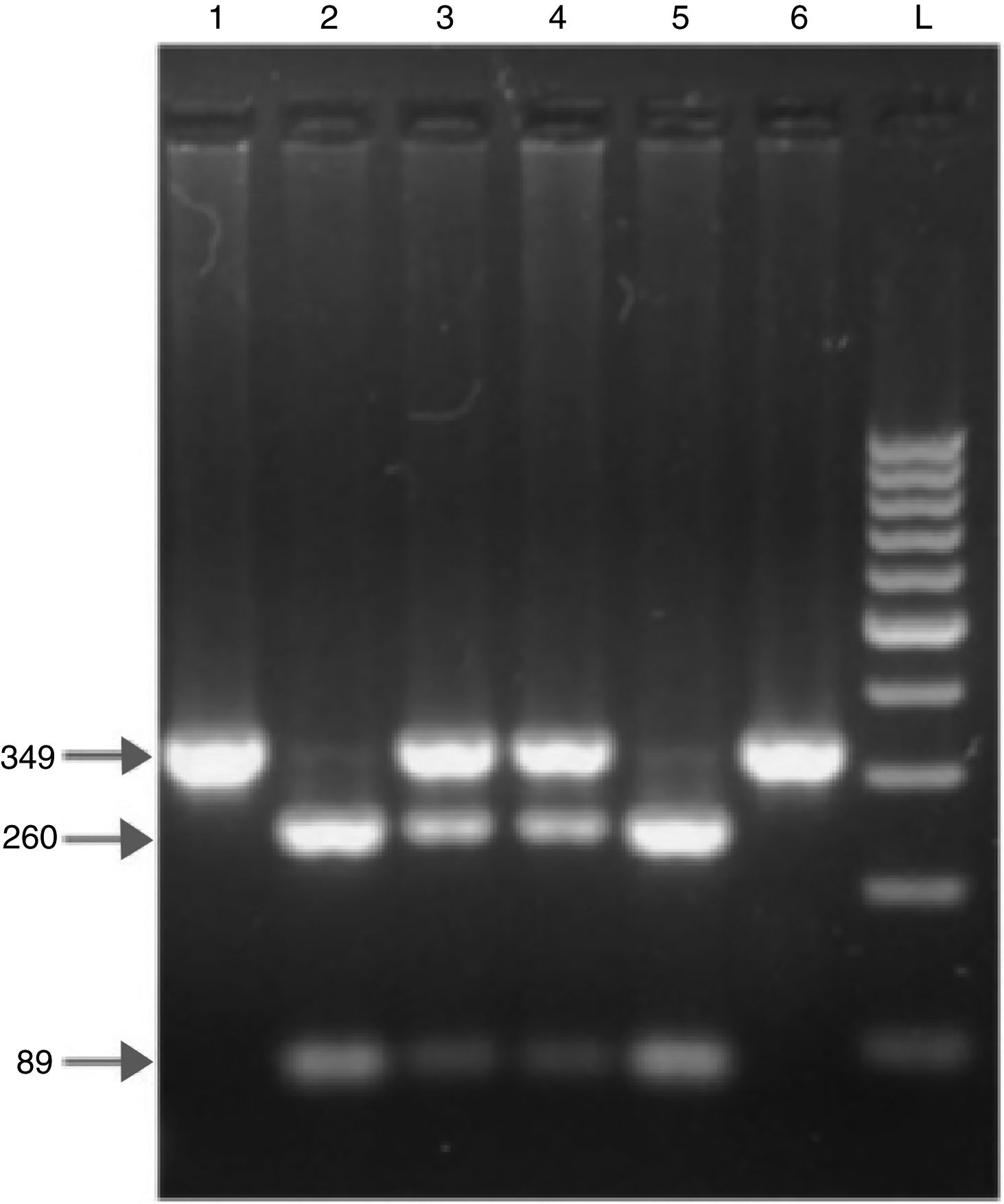
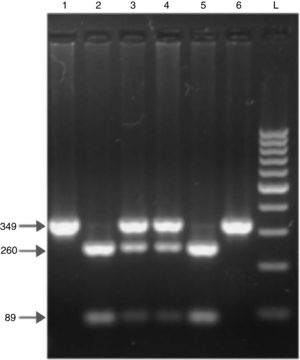
Blood samples were analyzed using enzyme-linked immunosorbent assay. PCR and restriction fragment length polymorphism were used for MBL2 genotyping. Serum MBL levels were measured using an immunoassay kit (Oligomer ELISA kit; Antibody Shop, Copenhagen, Denmark) according to the manufacturer instructions. The lowest detectable MBL concentration was 10ng/mL. For the definition of the functional MBL deficiency, this study used two different cut-off values of MBL concentration. An MBL level<700ng/mL was determined as deficiency and <150ng/mL as severe deficiency.1–3,18 DNA was extracted from the blood samples using a commercially available kit (Puregene, Gentra, MN, United States), and MBL2 genotyping was performed using these samples. DNA samples were maintained at −20°C until use. All genotypes were detected using PCR and restriction enzyme digestion. Exon 1 of MBL2 was amplified by PCR. The primer sequences were 5′-GTA GGA CAG AGG GCA TGC TC-3′ and 5′-CAG GCA GTT TCC TCT GGA AGG-3′. In all, a 349-bp PCR product was digested with BanI and MboII for codon 54 and codon 57, respectively. The normal allele (allele A) was cut into two fragments with BanI, 260 and 89bp. The variant allele B (rs1800450) and allele D (rs5030737) remained uncut. MboII cleaved the variant allele C (rs1800451) into 270 and 79bp fragments. The fragments were visualized using electrophoresis on 2% agarose gel. At electrophoresis, the dual band at the restriction site was defined as a heterozygous mutation, whereas the single band was defined as a homozygous mutation. As stated, the normal structural MBL2 allele was named A, whereas alleles B, C and D (mutation in codons 54, 57 and 52) were named O. A representative gel electrophoresis of the MBL2 exon 1 codon 54 polymorphisms is shown in Fig. 1.
DNA fragments on agarose gel electrophoresis after restriction enzyme digestion of exon 1 of the mannose-binding lectin (MBL2) gene codon 54. In all, 349bp PCR product was digested with BanI for codon 54 polymorphism. The normal allele (allele A) is cut into two fragments with BanI (lanes 2 and 5), 89 and 260bp. The variant allele (allele O) remains uncut (lanes 1 and 6). Both uncut and digested fragments are seen in AO heterozygote (lanes 3 and 4). L: 100bp DNA ladder.
This study was approved by the Ethics Committee of Uludag University Medical School and conformed to the standards set by the Declaration of Helsinki (15.01.2013-1/20). All parents provided informed consents prior to the inclusion of their children in the study.
Statistical analysisStatistical analysis was performed using SPSS v. 20.0 software (SPSS Inc., Chicago, IL, United States). The results are presented as median (interquartile range) for the variables showing non-Gaussian distribution and mean±standard deviation for data showing normal distribution. Student's t-test was used for group comparisons of normal distributions, and the Mann–Whitney U test was used for group comparisons of non-normal distributions. The chi-squared test and Fisher's exact test were used for the comparison of categorical variables. Logistic regression analysis was performed to investigate the effect of MBL2 genotype on RDS. The analysis included factors that were demonstrated in the literature to have an effect on RDS: gestational age, birth weight, sex, antenatal steroid administration, and MBL2 genotype were included in the analysis. A p-value of <0.05 was considered statistically significant.
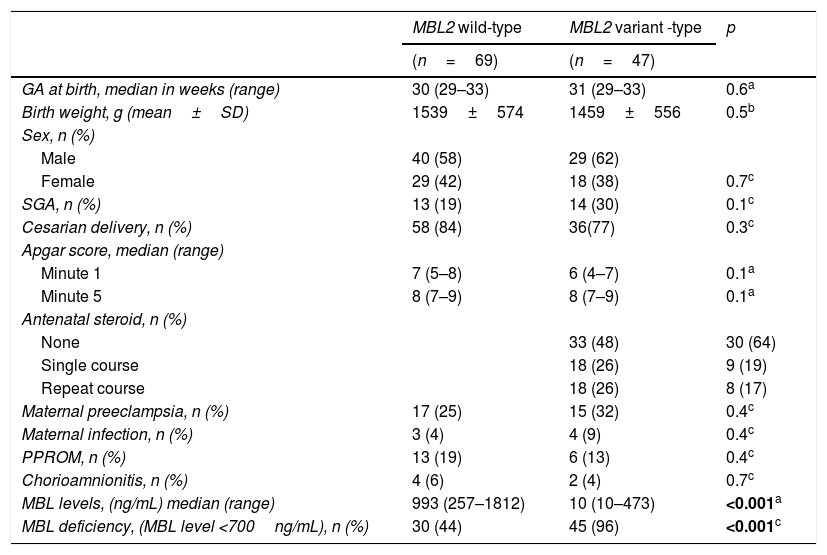
ResultsOverall, 131 preterm infants were included in this study. Ten were excluded because of blood sample insufficiency, four because of major congenital abnormalities, and one because of major surgery. In the final analysis, a total of 116 preterm infants were included: 69 with MBL2 wild-type (AA genotype) and 47 with MBL2 variant-type (AO/OO genotype). Overall, the rate of MBL2 variant-type in preterm infants was 41%. MBL levels were significantly lower and MBL deficiency and severe deficiency were higher in MBL2 variant-type than in MBL2 wild-type (p<0.001). Table 1 shows the demographic features of the study population. Codon 57 and 52 polymorphisms were not detected in any of the 116 preterm infants during the genetic evaluation. MBL2 codon 54 genotype and allele frequencies were 59% for MBL2 wild-type (AA genotype) and 41% for MBL2 variant-type (AO and OO genotype).
Neonatal and maternal characteristics of the study population.
| MBL2 wild-type | MBL2 variant -type | p | |
|---|---|---|---|
| (n=69) | (n=47) | ||
| GA at birth, median in weeks (range) | 30 (29–33) | 31 (29–33) | 0.6a |
| Birth weight, g (mean±SD) | 1539±574 | 1459±556 | 0.5b |
| Sex, n (%) | |||
| Male | 40 (58) | 29 (62) | |
| Female | 29 (42) | 18 (38) | 0.7c |
| SGA, n (%) | 13 (19) | 14 (30) | 0.1c |
| Cesarian delivery, n (%) | 58 (84) | 36(77) | 0.3c |
| Apgar score, median (range) | |||
| Minute 1 | 7 (5–8) | 6 (4–7) | 0.1a |
| Minute 5 | 8 (7–9) | 8 (7–9) | 0.1a |
| Antenatal steroid, n (%) | |||
| None | 33 (48) | 30 (64) | |
| Single course | 18 (26) | 9 (19) | |
| Repeat course | 18 (26) | 8 (17) | |
| Maternal preeclampsia, n (%) | 17 (25) | 15 (32) | 0.4c |
| Maternal infection, n (%) | 3 (4) | 4 (9) | 0.4c |
| PPROM, n (%) | 13 (19) | 6 (13) | 0.4c |
| Chorioamnionitis, n (%) | 4 (6) | 2 (4) | 0.7c |
| MBL levels, (ng/mL) median (range) | 993 (257–1812) | 10 (10–473) | <0.001a |
| MBL deficiency, (MBL level <700ng/mL), n (%) | 30 (44) | 45 (96) | <0.001c |
Values with significance are presented in bold.
MBL2, mannose-binding lectin; GA, gestational age; SGA, small for gestational age; PPROM, preterm premature rupture of membranes.
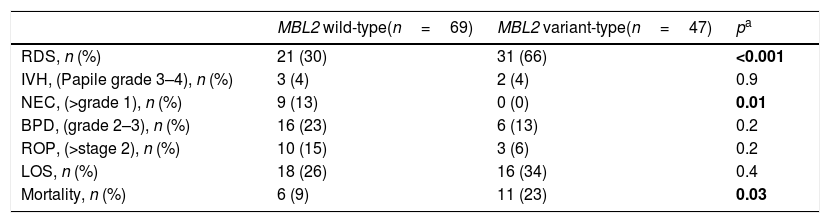
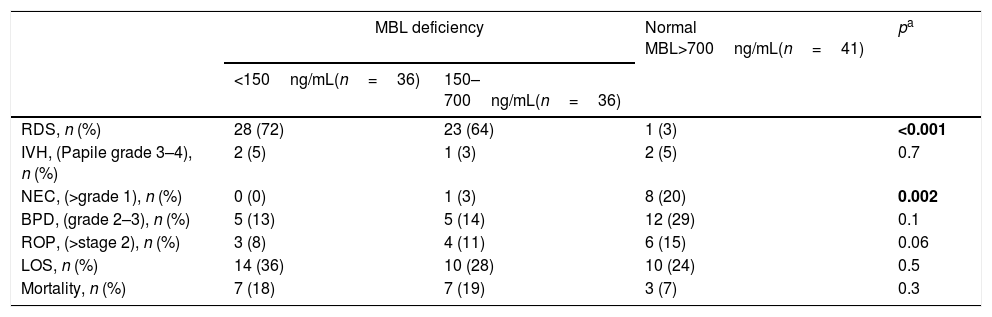
Evaluation of short-term morbidity based on MBL2 genotype revealed that RDS and mortality rates were significantly higher in the MBL2 variant-type group (p<0.001, p=0.03; respectively). NEC was found to be more prevalent in the MBL2 wild-type group (p=0.01). There was no difference between the MBL2 wild-type and variant-type groups in terms of IVH, BPD, ROP, and LOS (Table 2). Consideration of short-term morbidity based on MBL levels revealed that RDS was significantly higher in both the MBL deficient and severely deficient groups (p<0.001). NEC was found to be more common with normal levels of MBL (p=0.002). There was no significant difference in infants with or without MBL deficiency with respect to IVH, BPD, ROP, LOS, and mortality (Table 3). Further, because univariate analyses revealed that RDS development was more common in the MBL2 variant-type, the effect of gestational age, birth weight, gender, antenatal steroid use, and MBL2 genetics, which are factors that may affect RDS development, was investigated by logistic regression analysis. MBL2 variant-type was found to be an independent factor for the development of RDS (OR: 5.1, 95% CI: 2.2–11.9, p<0.001).
Frequency of early neonatal outcomes according to mannose binding lectin genotypes.
| MBL2 wild-type(n=69) | MBL2 variant-type(n=47) | pa | |
|---|---|---|---|
| RDS, n (%) | 21 (30) | 31 (66) | <0.001 |
| IVH, (Papile grade 3–4), n (%) | 3 (4) | 2 (4) | 0.9 |
| NEC, (>grade 1), n (%) | 9 (13) | 0 (0) | 0.01 |
| BPD, (grade 2–3), n (%) | 16 (23) | 6 (13) | 0.2 |
| ROP, (>stage 2), n (%) | 10 (15) | 3 (6) | 0.2 |
| LOS, n (%) | 18 (26) | 16 (34) | 0.4 |
| Mortality, n (%) | 6 (9) | 11 (23) | 0.03 |
Mannose binding lectin levels in relation to early neonatal outcomes.
| MBL deficiency | Normal MBL>700ng/mL(n=41) | pa | ||
|---|---|---|---|---|
| <150ng/mL(n=36) | 150–700ng/mL(n=36) | |||
| RDS, n (%) | 28 (72) | 23 (64) | 1 (3) | <0.001 |
| IVH, (Papile grade 3–4), n (%) | 2 (5) | 1 (3) | 2 (5) | 0.7 |
| NEC, (>grade 1), n (%) | 0 (0) | 1 (3) | 8 (20) | 0.002 |
| BPD, (grade 2–3), n (%) | 5 (13) | 5 (14) | 12 (29) | 0.1 |
| ROP, (>stage 2), n (%) | 3 (8) | 4 (11) | 6 (15) | 0.06 |
| LOS, n (%) | 14 (36) | 10 (28) | 10 (24) | 0.5 |
| Mortality, n (%) | 7 (18) | 7 (19) | 3 (7) | 0.3 |
Values with significance are presented in bold.
MBL, mannose-binding lectin; RDS, respiratory distress syndrome; LOS, late onset sepsis; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; BPD, bronchopulmonary dysplasia; ROP, retinopathy of prematurity.
It was observed that MBL levels were lower in preterm infants with MBL2 variant-type than in those with MBL2 wild-type. RDS was significantly more common in the MBL2 variant-type group and also in the MBL deficient group. Additionally, the mortality rates were higher in preterm infants with MBL2 variant-type. In the study model, MBL2 variant-type was a significant independent factor for RDS after adjusting for the effects of other factors. Besides, the prevalence of NEC was higher in the MBL2 wild-type group and with normal MBL levels. It is believed that these findings will contribute toward accumulating evidence on the effect of MBL in preterm morbidities.
The collectin family and MBL play an important role in the primary immune elimination of invasive microorganisms in the innate immune response as well as in the regulation of ongoing immune responses against microbial invasion. Studies have reported an association between MBL deficiency or variant genotype as well as infection and pulmonary pathologies.19 Pulmonary function impairment has been reported in patients with MBL deficiency and cystic fibrosis. Patients with bronchiectasis with MBL deficiency or variant-type have a higher rate of chronic microbial colonization and frequent recurrence of pulmonary problems.19,20 In some studies, similar to the results obtained in the present study, it has been shown that MBL deficiency or variant-type cause respiratory morbidity independent of infection.1,21 There is high sequence homology between MBL and SP-A and SP-D. The genes encoding these proteins are located on the long arm of chromosome 10 and belong to a similar lineage.22 SP-A and SP-D are involved in the removal of many pathogens in the lungs, and although SP-A is particularly known for its immune functions, RDS is associated with decreased SP-A levels.23 Mutant MBL2 genetics are associated with insufficient surfactant protein-A production, which may facilitate the development of RDS. Similarly, the present study also found a significant increase in RDS prevalence and mortality in patients with mutant MBL2 genetics. Early selective surfactant therapy in RDS has been reported to reduce pulmonary injury and mortality.24 It is believed that during the evaluation of MBL2 genotype at the time of delivery in premature infants with high RDS risk and ≤32 gestational weeks and in borderline cases with an indication for surfactant, the early administration of surfactants to patients with mutant MBL2 genetics will reduce mortality and pulmonary morbidities.
In recent years, there has been an increased interest in the association between MBL and inflammatory morbidities. It has been reported that MBL activates the lectin pathway of complement, resulting in ischemia-perfusion damage. In patients with MBL2 wild-type, higher MBL levels have been reported and associated with NEC, resulting in reperfusion injury after intestinal ischemia.10 In agreement with these findings, in the present study the prevalence of NEC was higher in preterm infants with MBL2 wild-type and normal MBL levels. However, some studies have reported that there is no association between MBL2 genotype and NEC.5,25 In the present work, the development of NEC may have been relatively more common because of significantly higher mortality rates in patients with MBL2 wild-type. Because there are debatable opinions in the literature on MBL2 genotype and NEC development, additional studies are needed to clarify this issue.
In this study, in agreement with previous data, no correlation was found between MBL2 genotype and MBL levels with inflammation-associated pathologies, BPD, IVH, and ROP.5,25 The evaluation of the association between MBL2 genotype and morbidity was the common aspect of these studies. It would be misleading to evaluate the association of MBL2 genotype and MBL value obtained at the time of delivery with morbidities alone. Because MBL levels increases as the gestational week increases in the MBL2 wild-type group, the evaluation of morbidity development with MBL levels obtained at different postnatal weeks may provide more accurate results to demonstrate the association between lectin pathway and inflammatory morbidities.2 There is a clear need for extensive studies to investigate the association between MBL and inflammatory morbidities in premature infants.
Although the association of MBL2 genotype with culture-proven sepsis has not been reported in the literature, the association between MBL2 genotype and early clinical sepsis has been reported.4,5,25 In contrast, the association between MBL deficiency and sepsis has been reported in many studies.3,18 In the present study, no association was found between MBL2 genotype and MBL levels with LOS. The authors believe that inadequate immune response to infection is observed because of low MBL levels in the early postnatal weeks in preterm infants even if the MBL2 genotype is wild-type. Therefore, future studies should evaluate MBL values at the time of sepsis together with genotype.
This study had some limitations. Morbidity was evaluated based on only MBL2 genotype and with the levels of MBL within 24h after birth because the MBL levels of the infants were not reassessed during the subsequent postnatal days. Additionally, the results obtained with a limited number of cases may not reflect the overall results. The strength of this study was that it evaluated the association of MBL2 genotype with MBL level and morbidities in preterm infants and simultaneously examined the MBL level in the first 24h of life.
In conclusion, the presence of MBL2 variant-type and low MBL levels are important risk factors for RDS development in preterm infants. Additionally, there is an association between MBL2 wild-type and NEC. Considering the importance of showing that MBL2 variant-type is an independent predictor of RDS, further prospective randomized studies on this topic are clearly required.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Dogan P, Ozkan H, Koksal N, Oral HB, Bagci O, Guney Varal I. Mannose-binding lectin gene polymorphism and its effect on short term outcomes in preterm infants. J Pediatr (Rio J). 2020;96:520–6.