To investigate the effectiveness of linezolid and vancomycin for the treatment of nosocomial infections in children under 12 years old.
Data sourcesThis is a systematic review in which five randomized clinical trials about the effectiveness of linezolid and vancomycin, involving a total of 429 children with nosocomial infections, were evaluated. They were searched in scientific databases: PubMed, Bvs, and SciELO.
Summary of findingsThe main nosocomial infections that affected children were bacteremia, skin, and soft tissue infections followed by nosocomial pneumonia. Most infections were caused by Gram-positive bacteria, which all studies showed infections caused by Staphylococcus aureus, with methicillin-resistant S. aureus (MRSA) and methicillin-resistant coagulase-negative staphylococci strains being isolated. Both linezolid and vancomycin showed high therapeutic efficacy against different types of nosocomial infections, ranging from 84.4% to 94% for linezolid and 76.9% to 90% for vancomycin. Patients receiving linezolid had lower rates of rash and red man syndrome compared to those receiving vancomycin. However, despite the adverse reactions, antimicrobials can be safely administered to children to treat nosocomial infections caused by resistant Gram-positive bacteria.
ConclusionBoth linezolid and vancomycin showed good efficacy in the treatment of bacterial infections caused by resistant Gram-positive bacteria in hospitalized children. However, linezolid stands out regarding its pharmacological safety. Importantly, to strengthen this conclusion, further clinical trials are needed to provide additional evidence.
A nosocomial infection (NI), also known as a healthcare-associated infection, refers to an infection acquired after a patient's admission to a hospital or other healthcare facility, which was neither present nor in the incubation period at the time of admission. These diseases are usually associated with invasive medical procedures, medical devices, or exposure to infectious agents in the hospital environment.1
Nosocomial hospitalizations represent a serious public health problem worldwide, as they can result in higher mortality rates, longer hospitalizations, and financial costs.2,3 The main pathogens associated with these infections are Staphylococcus aureus, coagulase-negative staphylococci (ECN), Escherichia coli, Enterococcus species, Klebsiella pneumoniae, and yeasts.4,5
Gram-positive bacteria can develop resistance to specific antibiotics.6 This resistance can occur intrinsically when the bacteria already have genetic information in their constitution that confers the ineffectiveness of the antimicrobial, or in an acquired way when the microorganism undergoes natural genetic attempts and recombinations.6,7
In this scenario, the environment and prevalence of drug-resistant Gram-positive bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) are becoming increasingly problematic in the treatment of NI in children.7 In this context, the use of vancomycin and linezolid has been increasingly frequent to combat these infections in pediatric patients.5,8
Vancomycin is a well-tolerated and effective glycopeptide antibiotic. However, its use in children admitted to the intensive care unit (ICU) can be challenging.9-11 It is estimated that most hospitalized pediatric patients with suspected coexisting severe staphylococci infection receive vancomycin as a first-line agent, due to the significant increase in MRSA infections reported in children's hospitals.11 Furthermore, vancomycin is often prescribed to treat infections caused by multidrug-resistant Gram-positive organisms.12,13 Its main spectrum of action covers MRSA, enterococci resistant to penicillin, and resistant strains of Streptococcus spp.10
Vancomycin acts through different controls, such as inhibition of peptidoglycan synthesis, alteration of cytoplasmic membrane permeability, and interference in cytoplasmic RNA synthesis.14 It is noteworthy that this antibiotic can cause some adverse effects. The most frequent include pain and phlebitis at the injection site. In addition, the "red man (or neck) syndrome", characterized by pruritus, erythema, congestion and angioedema in the neck and chest, may occur and may progress to shock. Another effect is ototoxicity, which can lead to irreversible hearing loss. Although rare, accumulation of vancomycin in the body can result in severe nephrotoxicity, leading to kidney failure.15,16 As a therapeutic alternative to vancomycin, linezolid has been shown to be effective and well-tolerated.13
Linezolid is a drug from the oxazolidinone group and was the first representative of this class to be approved for use. Its mechanism of action consists of inhibiting ribosomal proteins, through binding to the initiation site complex. Because it is a unique mechanism, linezolid does not show cross-resistance with other drug classes and demonstrates activity against resistant Gram-positive bacteria such as MRSA, penicillin-resistant pneumococci, and VRE.17
Linezolid is primarily used to treat hospital-acquired pneumonia, bacteremia, and infections caused by Gram-positive bacteria that are resistant to many drugs, including vancomycin.18,19 Importantly, linezolid is well tolerated both orally and intravenously. Major adverse effects include headache, diarrhea, nausea, and candidiasis. In addition, cases of anemia and thrombocytopenia have been observed, mainly related to the duration of treatment.15,20
It is essential to understand the appropriate antimicrobial prescription policy for a given environment, as well as the investigation of the responsible agents and their susceptibility profile to antimicrobials, in order to improve management and reduce the length of hospital stay.21 Thus, this study aimed to investigate the clinical efficacy of linezolid and vancomycin against nosocomial infections and their adverse reactions in children to provide scientific evidence for the medical community.
MethodsLiterature source and search strategyRandomized clinical trials were searched in scientific databases. Articles from the Virtual Health Library (VHL), National Library of Medicine (PubMed), and Scientific Electronic Library Online (SciELO) databases were used. The following MeSH (Medical Subject Headings) terms were used as a search strategy: linezolid; vancomycin; nosocomial infection, hospital-acquired Infections (HAI); newborn, child, and pediatrics.
The search for literature sources was carried out from December 2022 to January 2023. Eligible clinical studies that were published up to December 2022 and that evaluated the efficacy of linezolid or vancomycin in pediatric patients (<12 years) with nosocomial infections were included.
Health Sciences Descriptors (DeCS) corresponding to the terms of this review were used. In tracking the publications, the Boolean operators “AND” and “OR” were used, in order to combine the terms/descriptors mentioned above.
Study selectionTwo authors independently selected eligible clinical trials. Studies were included according to the following inclusion criteria: (1) Randomized clinical trials involving pediatric patients (< 12 years) with nosocomial infections; and (2) patients treated with linezolid or vancomycin not associated with other drugs. The authors did not place restrictions on race and year of publication due to scarcity in the literature on the topic involving this study population.
Trials that fell into the following categories were excluded: (1) treatment of patients outside the pediatric age range; (2) published in a language other than English; (3) duplicate literature, reviews, and case reports; (4) trials treating patients with other antibacterial agents in combination with linezolid or vancomycin; and (5) articles that were not available in full.
Data collectionThe following variables were collected from each included study: (1) year of publication, (2) study design, (3) study population, (4) clinical profile of patients, (5) duration of treatment, (6) pathogens associated with infections, (7) clinical cure rate after treatment with linezolid or vancomycin, and (8) adverse reactions.
The primary outcome evaluated was the resolution of the infection (clinical cure rate) with the use of the two drugs independently. Secondary outcomes included the prevalence of nosocomial visits, the main pathogens involved, and adverse reactions to linezolid or vancomycin. Clinical cure was defined as the decrease or disappearance of the main clinical signs and symptoms at the end of the treatment or at the follow-up visit to assess cure.
Finally, the data obtained were tabulated using the Microsoft Excel® program.
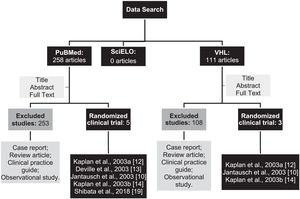
ResultsAfter searching the databases using the descriptors, 369 articles were identified. Among them, 258 were found in PubMed, 111 in the VHL, and no article was found in the SciELO database. Then, the studies were selected according to the eligibility criteria achieved, resulting in 3 duplicate articles in both PubMed and BvS (Figure 1).
The selected studies involved patients admitted to therapy with vancomycin or linezolid, regardless of dose, frequency, route of administration, and duration of treatment.
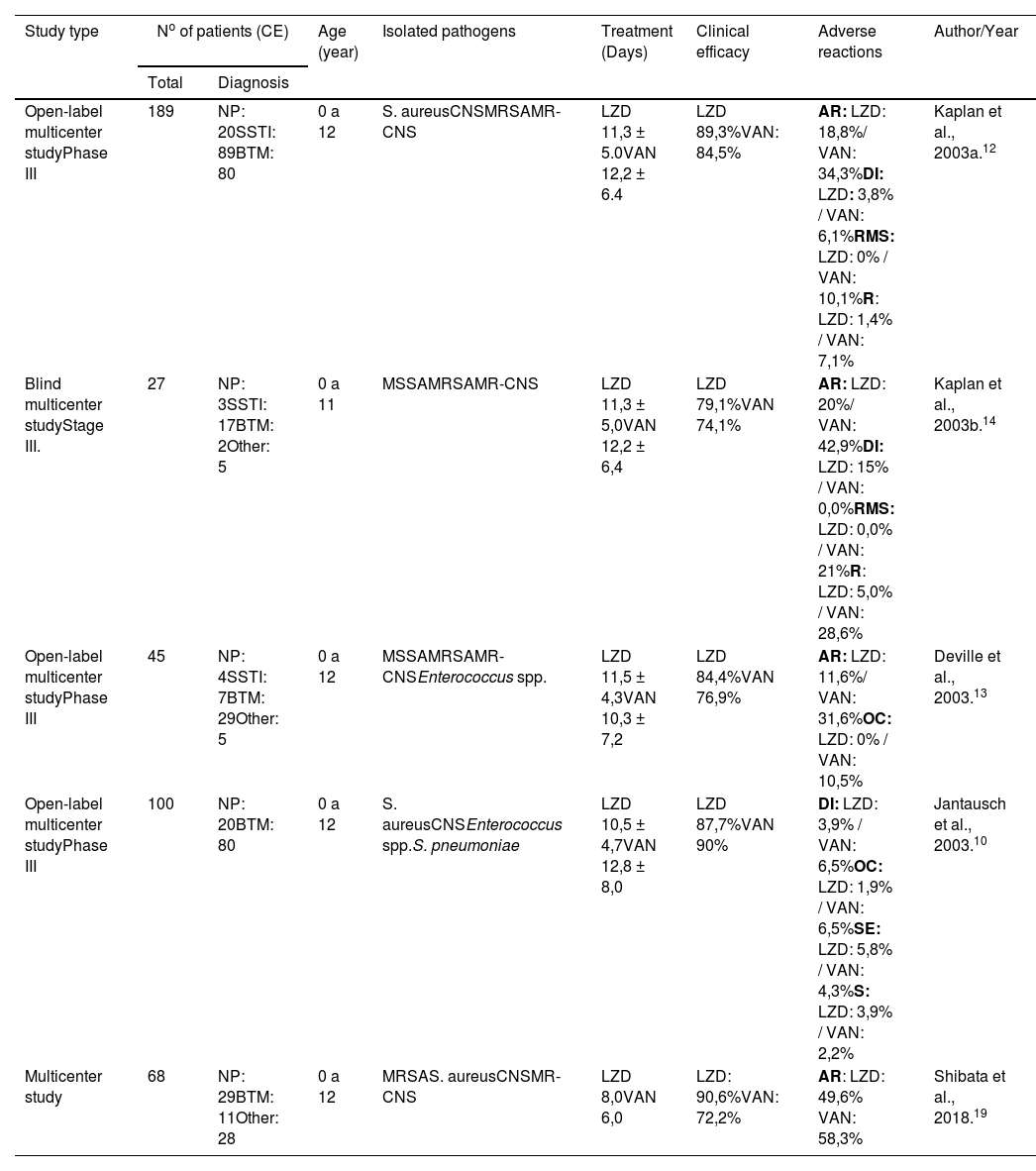
Considering the number of clinically available (CA) patients, the five randomized controlled clinical trials involved a total of 429 children with nosocomial infections. After collecting information from these studies, it was possible to assemble a comparative table showing the cure rates for both linezolid and vancomycin, in addition to age, type of pathogen, duration of treatment, and adverse reactions (Table 1).
Comparative analysis of clinical trials that evaluated the effectiveness of LZD and VAN in pediatric patients with nosocomial infection.
| Study type | No of patients (CE) | Age (year) | Isolated pathogens | Treatment (Days) | Clinical efficacy | Adverse reactions | Author/Year | |
|---|---|---|---|---|---|---|---|---|
| Total | Diagnosis | |||||||
| Open-label multicenter studyPhase III | 189 | NP: 20SSTI: 89BTM: 80 | 0 a 12 | S. aureusCNSMRSAMR-CNS | LZD 11,3 ± 5.0VAN 12,2 ± 6.4 | LZD 89,3%VAN: 84,5% | AR: LZD: 18,8%/ VAN: 34,3%DI: LZD: 3,8% / VAN: 6,1%RMS: LZD: 0% / VAN: 10,1%R: LZD: 1,4% / VAN: 7,1% | Kaplan et al., 2003a.12 |
| Blind multicenter studyStage III. | 27 | NP: 3SSTI: 17BTM: 2Other: 5 | 0 a 11 | MSSAMRSAMR-CNS | LZD 11,3 ± 5,0VAN 12,2 ± 6,4 | LZD 79,1%VAN 74,1% | AR: LZD: 20%/ VAN: 42,9%DI: LZD: 15% / VAN: 0,0%RMS: LZD: 0,0% / VAN: 21%R: LZD: 5,0% / VAN: 28,6% | Kaplan et al., 2003b.14 |
| Open-label multicenter studyPhase III | 45 | NP: 4SSTI: 7BTM: 29Other: 5 | 0 a 12 | MSSAMRSAMR-CNSEnterococcus spp. | LZD 11,5 ± 4,3VAN 10,3 ± 7,2 | LZD 84,4%VAN 76,9% | AR: LZD: 11,6%/ VAN: 31,6%OC: LZD: 0% / VAN: 10,5% | Deville et al., 2003.13 |
| Open-label multicenter studyPhase III | 100 | NP: 20BTM: 80 | 0 a 12 | S. aureusCNSEnterococcus spp.S. pneumoniae | LZD 10,5 ± 4,7VAN 12,8 ± 8,0 | LZD 87,7%VAN 90% | DI: LZD: 3,9% / VAN: 6,5%OC: LZD: 1,9% / VAN: 6,5%SE: LZD: 5,8% / VAN: 4,3%S: LZD: 3,9% / VAN: 2,2% | Jantausch et al., 2003.10 |
| Multicenter study | 68 | NP: 29BTM: 11Other: 28 | 0 a 12 | MRSAS. aureusCNSMR-CNS | LZD 8,0VAN 6,0 | LZD: 90,6%VAN: 72,2% | AR: LZD: 49,6% VAN: 58,3% | Shibata et al., 2018.19 |
AN, anemia; BTM, bacteremia; CA, clinically available; OC, oral candidiasis; S, seizure; DI, diarrhea; R, rash; CNS, coagulase-negative staphylococci; SSTI, skin and soft tissue infections; IV, intravenous; LZD, linezolid; MRSA, Methicillin-resistant Staphylococcus aureus; MSSA, Methicillin-susceptible S. aureus; MR-CNS, Methicillin-resistant coagulase-negative staphylococci; NP, nosocomial pneumonia; AR, adverse reaction; RMS, Red Man Syndrome; SE, sepsis; TP, thrombocytopenia; VAN, vancomycin; VO, vomit.
A multicenter study was conducted to compare the efficacy and safety of vancomycin and linezolid in the treatment of children with nosocomial pneumonia, complicated skin/skin structure infections, catheter-related bacteremia, bacteremia of unknown origin, or others caused by Gram-positive bacteria. Treatment with linezolid was initiated intravenously and, on average, after 3 days, it was switched to oral administration. In some cases, vancomycin was replaced by another orally administered drug. In addition, both groups received medication active against Gram-negative bacteria, such as aztreonam or gentamicin. Cure rates varied according to the type of infection: nosocomial pneumonia (linezolid = 90%, vancomycin = 100%); complicated skin/skin structure infections (linezolid = 93.2%, vancomycin = 90%); catheter-related bacteremia (linezolid = 84.8%, vancomycin = 80%); bacteremia of unknown origin (linezolid = 79.2%, vancomycin = 69.2%). In patients known or suspected to have infections caused by Gram-negative bacteria and who received coverage for this type of bacteria, clinical cure rates were generally lower in both treatment groups. The highest percentage of adverse events was observed with the use of vancomycin (34.3%; linezolid = 18.8%). The most common adverse effects were diarrhea (linezolid = 3.8% vs. vancomycin = 6.1%), red man syndrome (linezolid = 0% vs. vancomycin = 10.1%) and rash (linezolid = 1. 4% vs. vancomycin = 7.1%) (Table 1).12
In another study conducted on hospitalized children, aged 0 to 11 years, and diagnosed with pneumonia, bacteremia, or complicated skin and soft tissue infection caused by resistant Gram-positive pathogens, were given intravenous linezolid, with the option to switch to oral suspension (to the patients over 90 days old) or intravenous vancomycin. In the intention-to-treat (ITT) analysis, comprising 34 patients (20 receiving linezolid and 14 receiving vancomycin), all patients with MRSA infection were prescribed both linezolid and vancomycin, and the clinical cure rates were 94, 1% for linezolid and 90.0% for vancomycin. As for clinically available patients (n = 27), the clinical cure rate was 79.1% for linezolid and 74.1% for vancomycin. The most frequently reported adverse events in linezolid-treated patients with MRSA infections were diarrhea and thrombocytosis. The most frequently reported adverse events in vancomycin-treated patients with MRSA infections were rash, red man syndrome, and catheter site reactions (Table 1).14
In a phase III, randomized, open-label, controlled multicenter study to compare the efficacy, safety, and tolerability of linezolid compared to vancomycin in the treatment of bacterial antibiotics caused by resistant Gram-positive bacteria in children aged 0 to 12 years, a total of 63 neonates were included in the intention-to-treat analysis, with 43 of them treated with linezolid and 20 with vancomycin. In the intention-to-treat population, clinical cure rates were higher in the linezolid-treated group (77.5%; 31 of 40) compared with the vancomycin-treated group (61.1%; 11 of 18), although the differences were not statistically significant. For clinically available patients, clinical cure rates were 84.4% for linezolid and 76.9% for vancomycin. Regarding adverse reactions, the percentages of patients who experienced adverse events during the study were similar in both groups: 76.7% for patients treated with linezolid and 73.7% for patients treated with vancomycin. However, a smaller proportion of infants treated with linezolid reported drug-related adverse reactions compared to those treated with vancomycin (11.6% vs. 31.6% as shown in Table 1).13
In another study carried out on hospitalized children under 12 years, a 2:1 randomization process was performed for the use of linezolid or vancomycin. Patients received intravenous linezolid at a dose of 10 mg/kg every 8 hours, with the option of switching to linezolid as an oral suspension at the same dose and administration interval, or intravenous vancomycin at 10 to 15 mg/kg every 6 to 24 hours. Thirty-nine patients with pneumonia (23 receiving linezolid and 16 receiving vancomycin) and 113 patients with bacteremia (81 receiving linezolid and 32 receiving vancomycin) were included in the intention-to-treat (ITT) analysis. Clinical cure rates for clinically available patients with pneumonia did not differ between treatment groups (90.0% for linezolid and 100% for vancomycin). For patients with catheter-related bacteremia, no significant difference in clinical cure rates was observed between the linezolid and vancomycin groups (84.8% and 80.0%, respectively) in the clinically evaluated population. In this study, fewer patients treated with linezolid experienced drug-related adverse reactions compared to those treated with vancomycin (19.4% vs. 28.3%, as shown in Table 1).10
In a recent study, a total of 68 children were assigned to treatment with vancomycin and linezolid (32 received linezolid and 36 received vancomycin). The clinical cure rate was 90.6% for linezolid and 72.2% for vancomycin. Regarding adverse reactions, linezolid was less likely to cause such unwanted effects compared to vancomycin (49.6% for linezolid and 58.3% for vancomycin, according to Table 1). There was a significant decrease in platelet counts only in the linezolid group. However, no notable differences in safety were observed between the linezolid and vancomycin groups, even in neonates and infants.19
DiscussionThe present study confirmed the efficacy of both linezolid and vancomycin in the treatment of different nosocomial infections. The overall clinical efficacy between the two antimicrobials showed similar results, ranging from 84.4% to 94% for linezolid and from 76.9% to 90% for vancomycin (Table 1). These results corroborate previous studies and reinforce the effectiveness of these antibiotics in treating children, providing effective therapeutic options for severe hospital cases.
The study by Ye et al. (2020)22 used a systematic review and meta-analysis to investigate the efficacy and safety of vancomycin in the treatment of complications caused by MRSA in children. Results showed that vancomycin was effective in 87% of cases, with a treatment success rate of approximately 95%. In a recent study, in a recent study, Haseeb et al. (2021)23 performed a systematic review to investigate the efficacy of vancomycin in the treatment of infections caused by Gram-positive bacteria in critically ill patients, including children. Results revealed a positive therapeutic success rate when vancomycin was used, highlighting its clinical efficacy in treating critically ill children.
In another systematic review by Wu et al. (2017)24 the authors evaluated the effectiveness of linezolid in the treatment of infections caused by multidrug-resistant Gram-positive bacteria in children. The results indicated that linezolid was effective in about 85% of cases, demonstrating its clinical effectiveness in combating hospitalization in children. Another study by Ma et al. (2023)25 involved a systematic review and meta-analysis to assess the efficacy and safety of linezolid in children. The results indicated that linezolid achieved a therapeutic success rate of approximately 90%, demonstrating its effectiveness in the treatment of hospitalized children.
The present study demonstrated that the prevalence of the main nosocomial infections that affect children are bacteremia (BTM), skin and soft tissue infections (SSTI), followed by nosocomial pneumonia (NP). The prevalence of these infections was caused by Gram-positive bacteria, mainly: S. aureus, Enterococcus spp., S. pyogenes, and CNS. All studies showed infections caused by S. aureus, with MRSA and methicillin-resistant coagulase-negative staphylococci (MR-CNS) strains being isolated in most studies (Table 1).
The prevalence of nosocomial infections in pediatric patients is a concern in the health field. According to data from the National Nosocomial Infections Surveillance, the most frequently reported nosocomial infections in pediatric and neonatal intensive care units in the United States include pneumonia and bloodstream infections.26 Several studies reinforce the prevalence of infections found in the present study. Allegranzi et al. (2013),27 performed a systematic review to determine the prevalence of infections associated with health care in pediatric patients. The results revealed a prevalence of 7.1% of nosocomial infections in pediatric hospitals. Lower respiratory tract and urinary tract infections were the most common, followed by skin and soft tissue infections.
Zingg et al. (2015)28 performed a systematic review to examine the prevalence and risk factors of nosocomial infections in pediatric intensive care units in developing countries. The results revealed a wide variation in prevalence, from 3.8% to 37%. In addition, risk factors such as mechanical ventilation, intravenous catheters, and long hospital stays were associated with a higher risk of nosocomial infections.
It is important to note that the prevalence of specific pathogens may vary by geographic region, hospital setting, and antibiotic use practices.26 It is worth remembering that these nosocomial infections are associated with high morbidity and mortality.29,30 Generalized infection accounts for 25% of deaths in newborns requiring mechanical ventilation. Approximately 70% of all cases of severe sepsis in pediatric patients are reported in neonates, of which two quarters are low birth weight infants.31,32
Another relevant aspect to be considered in-hospital treatment is bacterial resistance.33 In recent years, there has been a worrying increase in the incidence of vancomycin resistance in several bacterial strains. A study by Howden et al. (2010)34 looked at vancomycin resistance in MRSA isolated from hospitals in different countries. Investigators observed a global spread of vancomycin-intermediate (VISA) and vancomycin-resistant (VRSA) MRSA clones. In another survey, investigators identified the presence of vancomycin resistance genes, such as vanA, vanB, and vanC, in different clinical isolates. They observed that these genes can be transferred between different bacterial species, which facilitates the spread of resistance.35,36
These data reinforce the need for new therapeutic alternatives that are effective against vancomycin-resistant bacteria. In this context, linezolid emerged as an alternative to vancomycin and gained Food and Drug Administration approval in 2022 for the treatment of children with pneumonia caused by Gram-positive cocci, skin or soft tissue infections, and vancomycin-resistant Enterococcus faecium infections.37 This approval highlights the importance of linezolid as an effective therapeutic option in the face of the growing challenge of vancomycin resistance.
In the present study, linezolid treatments had a lower frequency of adverse reactions in children with nosocomial infections than vancomycin (Table 1). Other studies corroborate these findings. A review conducted by Bruniera et al. (2015)16 highlighted several possible adverse reactions in children treated with vancomycin. Among these reactions, the authors reported cases of ototoxicity, nephrotoxicity and dermatological events. Although these reactions are relatively rare, it is critical to closely monitor pediatric patients receiving vancomycin and be alert for any signs of these reactions.
Regarding linezolid, a systematic review and meta-analysis by Shi et al. (2023)20 explored adverse reactions associated with the use of linezolid in children. The researchers found adverse events such as myelosuppression (decreased production of blood cells), peripheral neuropathy, and dermatologic reactions, including severe skin rashes. Importantly, myelosuppression can be a significant adverse reaction, particularly with long-term linezolid treatment in children. Therefore, regular hematological monitoring is recommended when using this antibiotic.
Both studies by Bruniera et al. (2015)16 and Shi et al. (2023)20 presented the importance of surveillance and monitoring of adverse reactions in children treated with vancomycin and linezolid. Furthermore, an analysis conducted by Beibei et al. (2010)38 on the safety of these two antibiotics in pediatric intensive care units also highlighted the need for preventive measures, such as periodic evaluation of renal and auditory function, as well as monitoring complete blood count during treatment.
Two limitations were identified in the present study. First, the sample used for this study was small (n = 429), making it necessary to carry out studies with larger cohorts. Second, only five studies were included, four of which were published 20 years ago, which may compromise the validity of current epidemiological data.
The present results indicate that both linezolid and vancomycin are highly effective in treating bacterial infections caused by resistant Gram-positive bacteria in children. However, the current analysis revealed that linezolid was shown to be safer compared to vancomycin. Patients treated with linezolid had lower rates of mask rash and red man syndrome compared with those treated with vancomycin. Therefore, linezolid can be considered a viable alternative to vancomycin in pediatric patients. However, it is important to emphasize that, due to the limited number of eligible articles included in this review (only 5), new clinical trials are needed to provide additional evidence and solidify this conclusion.
Final considerationsThis systematic review points to the efficacy of linezolid and vancomycin in the treatment of nosocomial bacterial infections in children. In addition, vancomycin has been widely used as a key treatment for severe infections caused by antibiotic-resistant Gram-positive pathogens in children. However, vancomycin has known toxicities, such as nephrotoxicity and, in less frequent cases, ototoxicity. Thus, it is necessary to regularly monitor drug levels, especially in patients with kidney problems, due to frequent use. Furthermore, it is important to note that vancomycin is only available in an intravenous form. Therefore, linezolid represents a viable option for the treatment of resistant Gram-positive bacteria, with the additional advantage of being available in an oral preparation.
PROPESP/UFPA supports the publication of this article.