This study aimed to evaluate factors associated with upper digestive hemorrhage and primary and secondary endoscopic prophylaxis outcomes in children with extrahepatic portal vein obstruction.
MethodsThis observational and prospective study included 72 children with extrahepatic portal vein obstruction who were followed from 2005 to 2017. Risk factors associated with upper digestive hemorrhage and the results of primary and secondary prophylaxis of these patients were evaluated.
ResultsFifty patients (69.4%) had one or more episodes of bleeding during follow-up, with a median age at first hemorrhage of 4.81 years. The multivariate analysis showed that medium- to large-caliber esophageal varices were associated with an 18-fold risk of upper digestive hemorrhage (95% CI: 4.33–74.76; p < 0.0001). Primary prophylaxis was administered to 14 patients, with eradication in 85.7%; however, 14.3% of these patients had hemorrhages during the follow-up period and 41.7% had a relapse of varices. Secondary prophylaxis was administered to 41 patients. Esophageal varices were eradicated in 90.2% of patients. There were relapse and re-bleeding of esophageal varices in 45.9% and 34.1% of the children, respectively.
ConclusionPrimary and secondary endoscopic prophylaxes showed high rates of esophageal varix eradication, but with significant relapses. Eradication of esophageal varices cannot definitively prevent recurrent upper digestive hemorrhage, since bleeding from alternate sites can occur. Medium- and large-caliber esophageal varices were associated with upper digestive hemorrhage in patients with extrahepatic portal vein obstruction. To the best of the authors’ knowledge, this study is the first to evaluate bleeding risk factors in children with extrahepatic portal vein obstruction.
Este estudo visou avaliar fatores associados à hemorragia digestiva alta e resultados da profilaxia endoscópica primária e secundária em crianças com obstrução extra-hepática da veia porta.
MétodosEste estudo observacional e prospectivo incluiu 72 crianças com obstrução extra-hepática da veia porta acompanhadas de 2005 a 2017.Os fatores de risco associados à hemorragia digestiva alta e os resultados da profilaxia primária e secundária desses pacientes foram avaliados.
ResultadosDos pacientes, 50 (69,4%) apresentaram ≥ 1 episódio de sangramento durante o acompanhamento, com idade média da primeira hemorragia de 4,81 anos. A análise multivariada mostrou que varizes esofágicas de médio a grande calibre estavam associadas a um risco 18 vezes maior de hemorragia digestiva alta (IC de 95% 4,33–74,76; p < 0,0001). Foi administrada profilaxia primária em 14 pacientes, com erradicação em 85,7%; contudo, 14,3% desses pacientes apresentaram hemorragias durante o período de acompanhamento e 41,7% apresentaram recidiva de varizes. Foi administrada profilaxia secundária em 41 pacientes. As varizes esofágicas foram erradicadas em 90,2% dos pacientes. Houve recidiva e novos sangramentos de varizes esofágicas em 45,9% e 34,1% das crianças, respectivamente.
ConclusãoAs profilaxias esofágicas primárias e secundárias apresentaram altas taxas de erradicação de varizes esofágicas, porém com recidivas significativas. A erradicação de varizes esofágicas não pode prevenir de forma definitiva a hemorragia digestiva alta recorrente, pois pode ocorrer sangramento de outros locais. Varizes esofágicas de médio e grande calibre estavam associadas à hemorragia digestiva alta em pacientes com obstrução extra-hepática da veia porta. No melhor de nosso conhecimento, nosso estudo é o primeiro a avaliar os fatores de risco de sangramento em crianças com obstrução extra-hepática da veia porta.
Extrahepatic portal vein obstruction (EHPVO) is a vascular disorder of the liver, comprising EHPVOs with or without intrahepatic or superior mesenteric and splenic venous involvement.1
Splenomegaly, hypersplenism, and the development of esophageal varices (EV) are the main manifestations of portal hypertension (PH) in EHPVO.2 EV rupture results in upper digestive hemorrhage (UDH), which occurs for the first time at a mean age of 3.0–5.3 years old.3–6 Upper digestive endoscopy (UDE) with sclerotherapy or variceal ligation is used to treat UDH and to administer primary and secondary prophylaxis.7
The Baveno VI Pediatric Satellite Symposium recommended that meso-Rex surgery should be performed whenever possible as a primary approach to EHPVO. However, there are anatomic and technical situations in which surgery cannot be performed. When meso-Rex shunt is not a viable option, the endoscopic approach can be used to provide secondary prophylaxis for UDH. Primary prophylaxis, although widely used in adults, is not well established for children with PH. The main reasons are: lack of data on the natural history of bleeding, limited data for defining the predictive endoscopic pattern of high risk of bleeding, and limited reports on the efficacy and safety of endoscopic prophylaxis in children.2
EHPVO is commonly approached from the perspective of UDH, extrapolating from studies of adults with liver cirrhosis. It is important to study pediatric patients in order to determine whether these extrapolations are appropriate. Thus, this study was undertaken to evaluate endoscopic factors associated with UDH and to investigate primary and secondary endoscopic prophylaxis outcomes in children with EHPVO. To the best of the authors’ knowledge, there are no other studies evaluating risk factors for EV bleeding exclusively in children with EHPVO.
Patients and methodsChildren with EHPVO (aged ≤ 18 years old at diagnosis) who were followed up in a tertiary hospital from January 2005 to December 2017 were included in the study. The protocol for the follow-up of pediatric PH was established in 2004, after approval by the Research Ethics Committee, and subsequently the laboratory and endoscopic clinical data were collected. Since then, new addenda and approvals have been included by the Research Ethics Committee. This series was followed during the routine clinical care by four main researchers who are responsible for the outpatient clinic and pediatric endoscopy sector.
The EHPVO diagnosis was confirmed through ultrasound with Doppler of the hepatic vessels. Thrombocytopenia and leukopenia were defined respectively as platelet count lower than 150,000 per mm3 and leukocyte count lower than 3000 per mm3. None of the patients had other associated liver diseases.
To evaluate factors associated with UDH, all the 72 patients were divided into two groups:
- -
Group with UDH: the first UDE of patients was performed during an acute UDH episode;
- -
Group without UDH: the first UDE was performed in patients without UDH.
Clinical and endoscopic data were collected during acute UDH episodes in the group with UDH. In the group without UDH, data were collected from the first UDE, performed as soon as the child had signs of PH. Continuous variables were categorized according to their reference values (platelets < 150,000 and international normalized ratio [INR] < 1.3).8,9 Platelets were categorized as < 50,000 and < 100,000, considered respectively as severe and moderate thrombocytopenia.9 Patients with incomplete data on the initial endoscopy were excluded from the analysis.
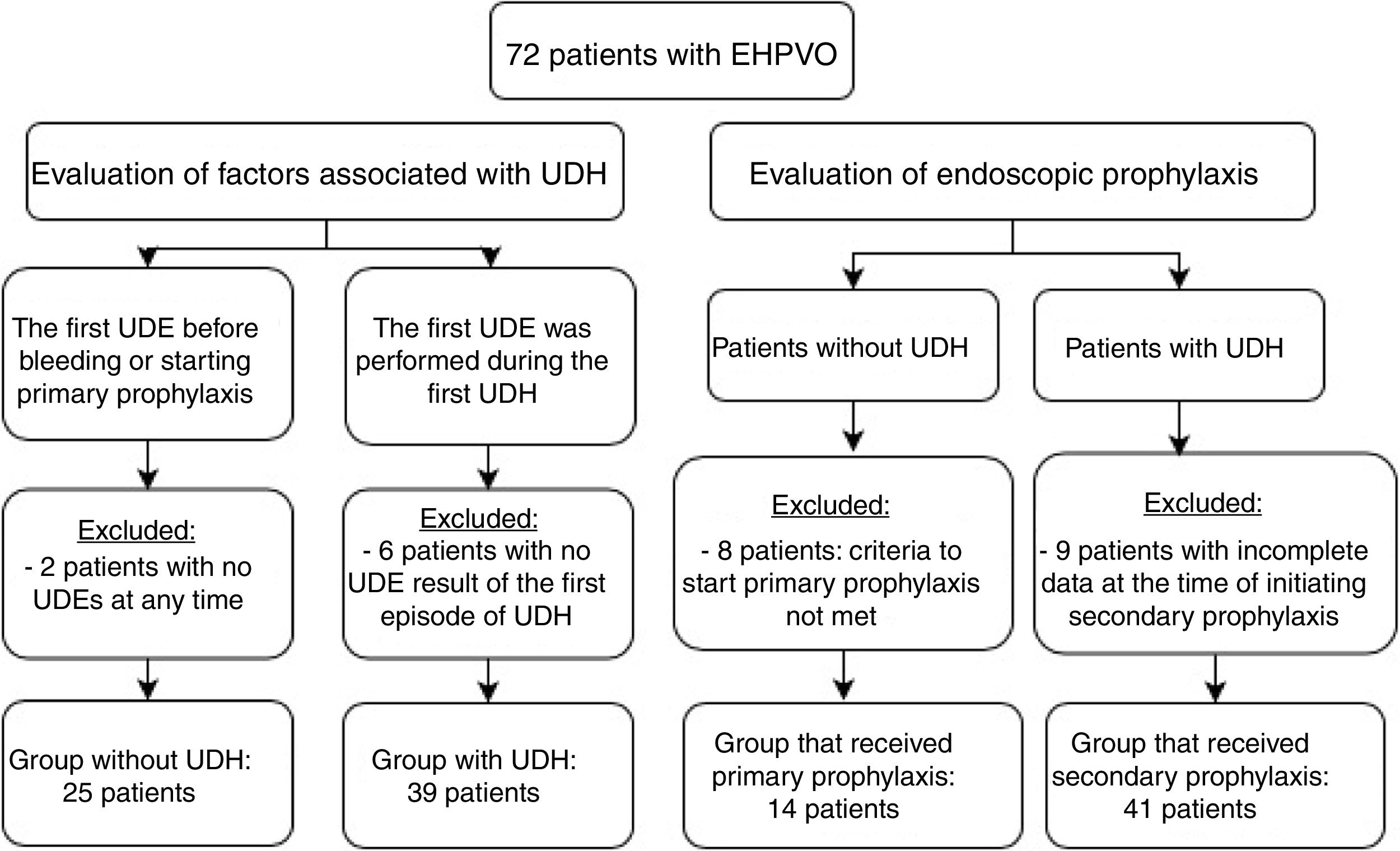
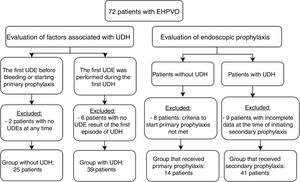
Primary and secondary prophylaxis clinical and endoscopic data were collected to analyze the following parameters: duration of follow-up, endoscopy, EV eradication, number of sessions required for eradication, EV relapse, number of UDH episodes, presence of previous gastric varices (GV) and portal hypertension gastropathy (PHG), and onset of GV and PHG. Patients whose data from endoscopies was impossible to recover and patients from primary prophylaxis without criteria to start it were excluded from this analysis. Fig. 1 shows the number of patients in each analysis and indicates the reasons for exclusion from specific evaluations.
This study was approved by the Research Ethics Committee of the institution (COEP No. 254/04, 258/09, and 474/09; CAAE No. 460087316.2.0000.5149). Informed consent and participation agreement forms were signed by all participants.
ProtocolAfter managing the acute UDH secondary to EV, patients were referred for a secondary endoscopic prophylaxis method – either sclerotherapy (ST) or elastic ligation of esophageal varices (ELEV) – to be performed after a two-week interval. The endoscopies in the hospital are performed by three experienced pediatric endoscopists and during the exams, two of them are always present. EV were ranked according to the Japanese classification system: small caliber (F1): small varicose veins, not tortuous; medium caliber (F2): moderately enlarged and tortuous varices; large caliber (F3): markedly enlarged and nodular varicose veins.10 The EV with the largest caliber was used for classification.
GVs were classified as esophagogastric varices (EGV) with lesser curvature extension (type EGV1), esophagogastric varices extending to the gastric fundus (type EGV2), isolated gastric fundal varices (IGV1), or gastric fundal and/or duodenal varices (IGV2).11 Red spots, PHG, and other mucosal lesions were identified during each endoscopic examination.12,13
During primary and secondary prophylaxis periods, patients underwent endoscopic procedures every three weeks until the varices were eradicated. After eradication, UDEs were performed at quarterly intervals for the first six months, then once every six months and, among those who remained free of varices, once a year thereafter. UDEs were performed any time UDH was suspected or diagnosed. Primary prophylaxis with ELEV was recommended for patients who had thin-caliber EV with red spots, medium- or large-caliber varices, or varices extending to the gastric cardia. Beta-blockers were not used for prophylaxis in this service because their efficacy and safety for use in children with PH has not been established.
Definition of terms (studied variables)Several terms are continuously referred to in this study and represent the variables evaluated in the analysis. Those terms are:
Eradication: all visible varices that were thrombosed through ST, were too thin to be suctioned through ELEV, or were absent;
Re-bleeding: an episode of UDH after the initiation of prophylaxis, with clinical repercussions requiring urgent UDE, divided into two time periods:
Early: occurrence before eradication (not associated with complications of the endoscopic procedure);
Late: after eradication;
Relapse: re-emergence of EV requiring endoscopic management in a patient who had previously experienced eradication;
Emergence of PHG: onset of PHG in a patient whose first UDH did not occur prior to prophylaxis;
Emergence of GV: onset of gastric fundal varices in a patient whose first UDH did not occur prior to prophylaxis.
Statistical analysisThe database was analyzed using IBM SPSS Statistics®, v. 20.0 (IBM Corp., Armonk, NY, United States). Continuous variables without normal distributions were compared using the non-parametric Mann-Whitney U-test. Continuous variables with normal distributions were compared using Student’s t-test. Categorical variables were compared using Pearson’s asymptotic chi-squared test (when < 20% of the expected values were between 1 and 5) and Pearson’s exact chi-squared test (when > 20% of the expected values were between 1 and 5). Results were considered significant when less than 0.05 (p ≤ 0.05). When categorical variables were significant according to chi-squared tests and the variables had more than two standards, standardized adjusted residual values were considered to be significantly different when the residual values were ≥ +1.96 and ≤ −1.96.
To identify factors associated with UDH, a multivariate analysis was conducted using the logistic regression method. All variables with p-values < 0.20 in the univariate analysis were included in the multivariate analysis. The logistic regression model was adapted so that all variables were considered significant at 0.20. Variables with higher p-values were discarded until the final model showed all significant variables at 0.05. The association measure used was the relative risk (RR), which was estimated by the odds ratio (OR) and a confidence interval of 95% for variables associated with the first episode of UDH. The adjustment quality was evaluated using the Hosmer & Lemeshow test.
ResultsOf the 72 patients analyzed, 37 (51.4%) were boys. The median age at the time the first sign or symptom of EHPVO appeared was 2.65 years (Q1; Q3: 1.08; 5.02). Splenomegaly with hypersplenism was the initial manifestation in 48.6% of these patients, followed by UDH in 43% and abdominal pain in 5.6%. The median age at the time of the first consultation at the facility was 4.79 years (Q1; Q3: 2.38; 8.54). One patient died during follow-up due to a severe episode of UDH. The mean duration of follow-up for these patients was 8.90 ± 5.60 years.
Umbilical catheterization, the main causative factor identified in these patients, occurred in 31 (43.1%), followed by neonatal sepsis in 23 (31.9%). Other risk factors identified were: thrombophilia in five patients (6.9%), congenital malformations in three (4.2%), abdominal infections in three (4.2%), dehydration in three (4.2%), and abdominal surgery in two (2.8%). There were no risk factors identified in 30 of the patients (41.7%). There were 19 patients (26.4%) with only one risk factor identified, while 18 (25%) and five (6.9%) had, respectively, two and three risk factors identified.
Persistent thrombocytopenia was detected in 88.9% of the patients and 37.5% had leukopenia. In 94.4% of patients, EVs were identified during follow-up. PHG, GV, and portal hypertensive duodenopathy occurred in 88.9%, 80.6%, and 4.2% of patients, respectively.
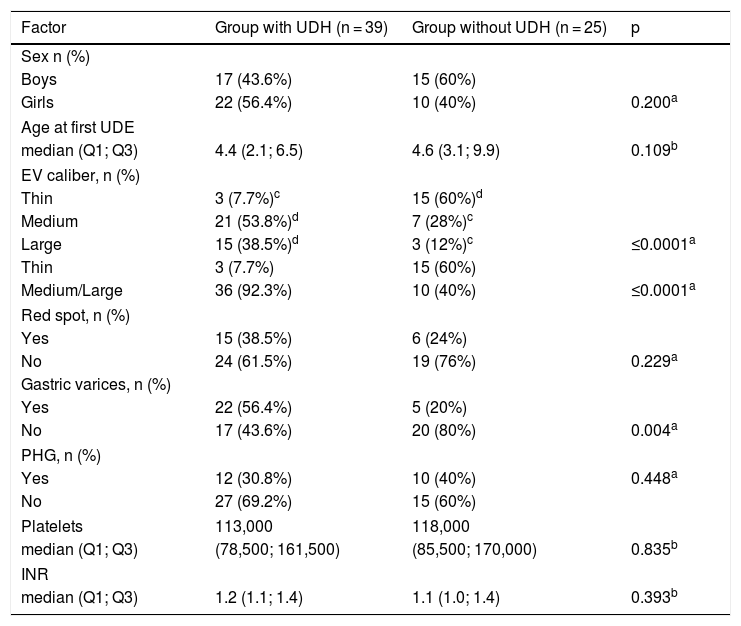
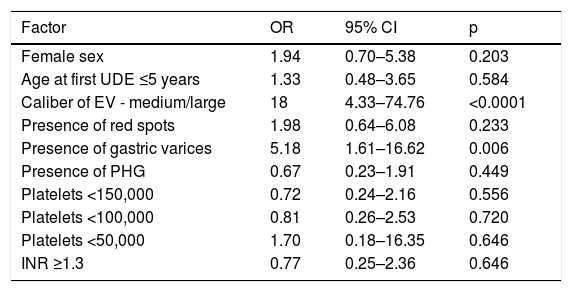
To evaluate the risk factors associated with the initial UDH episode, the patients were divided into two groups and their data were analyzed. Data of both groups are shown in Table 1. After the univariate analysis was completed (data shown in Table 2), GV and EV were evaluated in the multivariate logistic regression analysis. After the multivariate analysis, the presence of medium- and large-caliber EV was the only factor associated with UDH, with an OR of 18 and 95% CI of 4.33–74.76 (p < 0.0001).
Comparison of clinical, endoscopic, and laboratory factors related to UDH episodes: UDH group vs. non-UDH group (n = 64).
| Factor | Group with UDH (n = 39) | Group without UDH (n = 25) | p |
|---|---|---|---|
| Sex n (%) | |||
| Boys | 17 (43.6%) | 15 (60%) | |
| Girls | 22 (56.4%) | 10 (40%) | 0.200a |
| Age at first UDE | |||
| median (Q1; Q3) | 4.4 (2.1; 6.5) | 4.6 (3.1; 9.9) | 0.109b |
| EV caliber, n (%) | |||
| Thin | 3 (7.7%)c | 15 (60%)d | |
| Medium | 21 (53.8%)d | 7 (28%)c | |
| Large | 15 (38.5%)d | 3 (12%)c | ≤0.0001a |
| Thin | 3 (7.7%) | 15 (60%) | |
| Medium/Large | 36 (92.3%) | 10 (40%) | ≤0.0001a |
| Red spot, n (%) | |||
| Yes | 15 (38.5%) | 6 (24%) | |
| No | 24 (61.5%) | 19 (76%) | 0.229a |
| Gastric varices, n (%) | |||
| Yes | 22 (56.4%) | 5 (20%) | |
| No | 17 (43.6%) | 20 (80%) | 0.004a |
| PHG, n (%) | |||
| Yes | 12 (30.8%) | 10 (40%) | 0.448a |
| No | 27 (69.2%) | 15 (60%) | |
| Platelets | 113,000 | 118,000 | |
| median (Q1; Q3) | (78,500; 161,500) | (85,500; 170,000) | 0.835b |
| INR | |||
| median (Q1; Q3) | 1.2 (1.1; 1.4) | 1.1 (1.0; 1.4) | 0.393b |
UDH, upper digestive hemorrhage; UDE, upper digestive endoscopy; EV, esophageal varices; PHG, portal hypertension gastropathy; INR, international normalized ratio.
Assessment of factors related to the first episode of UDH according to the univariate analysis.
| Factor | OR | 95% CI | p |
|---|---|---|---|
| Female sex | 1.94 | 0.70–5.38 | 0.203 |
| Age at first UDE ≤5 years | 1.33 | 0.48–3.65 | 0.584 |
| Caliber of EV - medium/large | 18 | 4.33–74.76 | <0.0001 |
| Presence of red spots | 1.98 | 0.64–6.08 | 0.233 |
| Presence of gastric varices | 5.18 | 1.61–16.62 | 0.006 |
| Presence of PHG | 0.67 | 0.23–1.91 | 0.449 |
| Platelets <150,000 | 0.72 | 0.24–2.16 | 0.556 |
| Platelets <100,000 | 0.81 | 0.26–2.53 | 0.720 |
| Platelets <50,000 | 1.70 | 0.18–16.35 | 0.646 |
| INR ≥1.3 | 0.77 | 0.25–2.36 | 0.646 |
UDH, upper digestive hemorrhage; OR, odds ratio; CI, confidence interval; UDE, upper digestive endoscopy; EV, esophageal varices; PHG, portal hypertension gastropathy; INR, international normalized ratio.
Fifty patients (69.4%) had at least one episode of UDH during the follow-up period. The median age at the time of the first bleeding episode was 4.81 (Q1; Q3: 2.09; 7.34). These patients were recommended to start secondary prophylaxis. Nine patients were excluded from the secondary prophylaxis analysis because of incomplete data from endoscopy. Of 22 patients without UDH, 14 were recommended to start primary prophylaxis, according to the criteria already described, and were referred for endoscopy. The results of the analysis of these two groups are shown in Table 3.
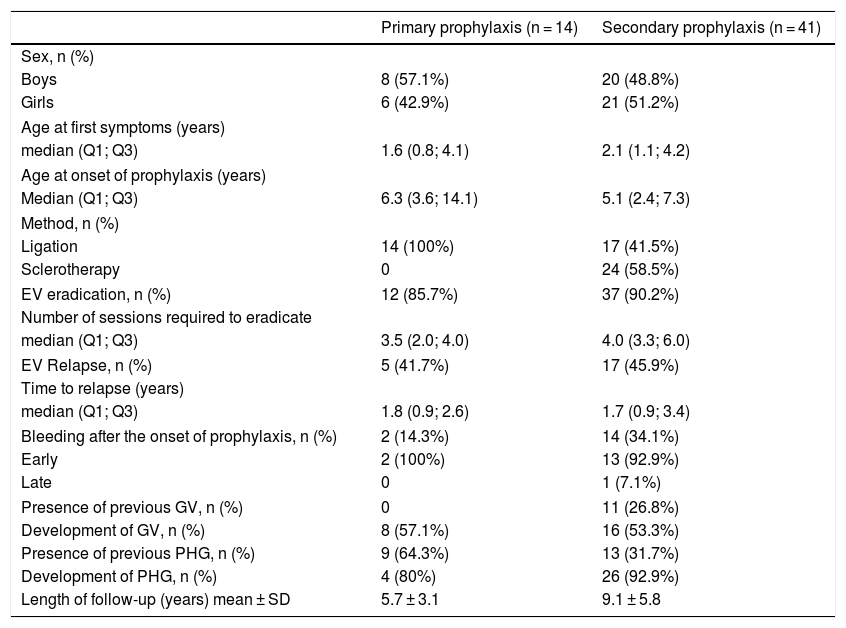
Characteristics of patients who received primary and secondary endoscopic prophylaxis (n = 55).
| Primary prophylaxis (n = 14) | Secondary prophylaxis (n = 41) | |
|---|---|---|
| Sex, n (%) | ||
| Boys | 8 (57.1%) | 20 (48.8%) |
| Girls | 6 (42.9%) | 21 (51.2%) |
| Age at first symptoms (years) | ||
| median (Q1; Q3) | 1.6 (0.8; 4.1) | 2.1 (1.1; 4.2) |
| Age at onset of prophylaxis (years) | ||
| Median (Q1; Q3) | 6.3 (3.6; 14.1) | 5.1 (2.4; 7.3) |
| Method, n (%) | ||
| Ligation | 14 (100%) | 17 (41.5%) |
| Sclerotherapy | 0 | 24 (58.5%) |
| EV eradication, n (%) | 12 (85.7%) | 37 (90.2%) |
| Number of sessions required to eradicate | ||
| median (Q1; Q3) | 3.5 (2.0; 4.0) | 4.0 (3.3; 6.0) |
| EV Relapse, n (%) | 5 (41.7%) | 17 (45.9%) |
| Time to relapse (years) | ||
| median (Q1; Q3) | 1.8 (0.9; 2.6) | 1.7 (0.9; 3.4) |
| Bleeding after the onset of prophylaxis, n (%) | 2 (14.3%) | 14 (34.1%) |
| Early | 2 (100%) | 13 (92.9%) |
| Late | 0 | 1 (7.1%) |
| Presence of previous GV, n (%) | 0 | 11 (26.8%) |
| Development of GV, n (%) | 8 (57.1%) | 16 (53.3%) |
| Presence of previous PHG, n (%) | 9 (64.3%) | 13 (31.7%) |
| Development of PHG, n (%) | 4 (80%) | 26 (92.9%) |
| Length of follow-up (years) mean ± SD | 5.7 ± 3.1 | 9.1 ± 5.8 |
EV, esophageal varices; GV, gastric varices; PHG, portal hypertension gastropathy; SD, standard deviation.
In the group of patients who received secondary prophylaxis, the sites of the primary UDH episode were: EV in 84.4% of UDH in which UDE was performed before prophylaxis commenced, followed by GV in 6.7%, and PHG in 2.2%. After eradicating EV, re-bleeding occurred primarily in GV in 47.2% of patients, followed by PHG in 8.3%, and EV in 8.3% of UDH in which UDE was performed. The bleeding site could not be identified on endoscopy in 6.7% of patients prior to the onset of prophylaxis and in 36.1% of patients after EV eradication. After the onset of secondary prophylaxis, 78%, 71%, and 68% of patients remained without new bleeding from EV after one, five, and ten years of follow-up, respectively. After EV eradication, 97%, 92%, and 89% of the children remained without EV bleeding at one, five, and ten years of follow-up, respectively. After initiation of primary prophylaxis, 86% of patients had no new episodes of EV bleeding at one, five, and ten years of follow-up, as the episodes occurred soon after initiation of prophylaxis.
Regarding endoscopic treatment complications, four children developed esophageal stenosis after four to ten sessions, three of them underwent ST, and one underwent ELEV. Mechanical dilatation was required in three patients. In two, the elastic bands fell off less than 24 h after ELEV, followed by episodes of severe UDH.
Two patients required urgent surgery due to severe UDH after secondary prophylaxis with sclerotherapy was initiated. This was considered a failure of endoscopic treatment. Both patients underwent azygos-portal disconnection, which was the choice of the pediatric surgeon for uncontrolled bleeding emergency. None of the patients underwent meso-Rex shunt procedures, because it was not available in this hospital.
DiscussionAlthough EHPVO is a primary cause of PH in children, few studies have investigated this group of patients. EHPVO is commonly approached from the perspective of UDH, extrapolating from studies of patients with liver cirrhosis. Thus, it is important to understand its natural history in pediatric patients, to determine whether these extrapolations are appropriate. To the best of the authors’ knowledge, this study is the first to evaluate EV bleeding risk factors in children and adolescents with EHPVO. It was found that medium- and large-caliber EV conferred an 18-fold increased bleeding risk with UDH in patients with EHPVO. Endoscopic prophylaxes led to high rates of EV eradication, but the number of relapses was significant. EV eradication cannot definitively prevent recurrent UDH since alternate-site bleeding can occur, which underlines the need for adequate follow-up.
According to the literature, for 40–64% of pediatric patients, the first manifestation of EHPVO is UDH. The age at first bleeding episode is reported to lie between 3.0 and 5.3 years old.3–6,14 These data were confirmed in this study, reinforcing the data on the natural history of EHPVO already described. Throughout the follow-up period, 50 patients (69.4%) had at least one episode of UDH, which emphasizes the need to establish appropriate approach.
Pediatric studies of children with cirrhosis are scarce and most have analyzed patients with cirrhosis secondary to biliary atresia (BA). In 2010, Duché et al. analyzed 139 children with BA and concluded that the endoscopic risk factors for UDH included the following: large-caliber varices, variceal red spots, and variceal extension to the cardia.15 In 2017, the same authors analyzed 1300 children with PH who were divided into two groups: a BA group and a group with PH of various etiologies (including 155 with EHPVO). They concluded that endoscopic signs indicating bleeding risk were similar in both groups and included medium- and large-caliber varices, red spots, and GV extending to the cardia. However, they did not analyze the patients with EHPVO separately, but rather included them in a group with several other etiologies for PH.16
Pediatric studies that assessed risk factors for bleeding in PH analyzed children with cirrhosis, such as Wanty et al. in 2013, who studied 83 children with BA. Their multivariate analysis showed that large-caliber EV, red spots, and low fibrinogen values were associated with an increased risk of UDH.17 Pimenta et al. evaluated 103 children with cirrhosis from several causes. Their multivariate analysis showed that GV and red spots on EV were associated with UDH.18
In the present study’s multivariate analysis, medium- to large-caliber EV were associated with an 18-fold increased risk of bleeding and UDH, similar to that described in children with cirrhosis. Despite the large confidence interval, the OR was high, reflective of a considerable risk of bleeding in patients with medium- or large-caliber varices. GV was associated with UDH in the univariate analysis, but lost significance after the multivariate analysis. Sample size may have been a limiting factor in the GV analysis. It is important to understand these risk factors when analyzing the indications for and completion of primary prophylaxis, to avoid/reduce the risk of UDH. Although thrombocytopenia is a good indicator of PH, its presence was not associated with the risk of bleeding in this study or in other studies in children with PH caused by cirrhosis.
Primary endoscopic prophylaxis in pediatrics is described in very few studies and is even more rare in studies of patients with EHPVO.19–22 In 2009, Maksoud-Filho et al. evaluated 32 patients with EHPVO who received primary prophylaxis with ST and observed that none of them had UDH after completing prophylaxis therapy.21 Other studies19,20,22 that evaluated children with EHPVO and concomitant PH secondary to intrahepatic causes reported EV eradication rates of 90% to 94%. Among patients who underwent ELEV, there was no bleeding throughout the follow-up period,22 whereas the patients that underwent ST had bleeding rates ranging from 24% to 42.3%.19,20
The finding of a significant number of variceal relapses reinforces the Baveno VI Pediatric Satellite Symposium recommendations,2 which encourage the use of a meso-Rex shunt whenever possible as a primary approach to EHPVO. When the surgery is not possible, primary endoscopic prophylaxis is important and efficient for reducing UDH episodes, although periodic follow-up is necessary.
Secondary endoscopic prophylaxis in children with EHPVO is associated with a high EV eradication rate.3,21–30 Nevertheless, the rates of variceal relapse and the need for repeat sessions were slightly higher than those reported in previous studies of patients with EHPVO, ranging from 6.6% to 40%.3,24,26–30 The rate of re-bleeding observed in the present study was higher in comparison with rates reported in the literature, with values ranging from 3% to 27.8%.3,21,23–30
Eradication of EV does not guarantee the absence of new UDH, as several patients started having bleeding from other sites. In the present sample, nearly 90% of patients developed PHG during follow-up and 80% had GV. With the increased prevalence of PHG and GV, there was a change in the focus of UDH in this group of patients.
Similarly to other studies evaluating EHPVO, a limitation of this study is the patient sample size, which limits extrapolations or definitive conclusions; however, the prospective cohort study design provides consistency in the findings. Another aspect that is worth discussing as a limitation is the characteristics of the varices during or near the bleeding episode. Due to hemodynamic changes caused mainly by blood loss, the classification of varicose veins may be underestimated, representing a limitation of the type of study performed.
In conclusion, it was observed that medium- and large-caliber EV were associated with the risk of UDH in patients with EHPVO, with an 18-fold increase in the chance of bleeding. These findings followed the same trend as studies on cirrhotic patients, such that the size of the varices was a relevant factor associated with bleeding risk. Endoscopic prophylaxis showed high rates of EV eradication; however, a significant number of relapses occurred, which emphasizes the need for long-term follow-up. Furthermore, EV eradication could not guarantee the absence of new-onset UDH because there was an increased risk of bleeding from other sites. Further studies including a larger number of patients with EHPVO are required to develop reliable conclusions.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Oliveira AP, Ferreira AR, Fagundes ED, Queiroz TC, Carvalho SD, Neto JA, et al. Endoscopic prophylaxis and factors associated with bleeding in children with extrahepatic portal vein obstruction. J Pediatr (Rio J). 2020;96:755–62.