To systematically review evidence related to nutritional and cardiometabolic outcomes in children born at term and small for gestational age and the association with breastfeeding.
Source of dataTwo independent reviewers searched the MEDLINE, LILACS, SciELO, and Embase databases without time or language restrictions. The PRISMA tool was used, and studies that evaluated infants born at term and small for gestational age, breastfed, and with an evaluation of cardiometabolic outcomes were included. Studies with preterm infants, those that did not have information on breastfeeding, and those with lack of evaluation of the outcome variables were excluded. Also excluded were review articles, editorials, and series of cases.
Summary of dataOnly seven articles were found that met the abovementioned criteria. There was a great variability in the type of evaluation, as well as in the age of these children. It was demonstrated that breastfeeding promoted growth without body composition alteration and without increased insulin resistance in children with exclusive breastfeeding, when compared to children receiving a higher calorie formula, except for one article that observed an increase in fat mass in exclusively breastfed children.
ConclusionBreastfeeding seems to be a safe feeding practice for infants born at term and small for gestational age, showing no association with deleterious short-term outcomes. Breastfeeding stimulation in these populations seems to be a way of preventing the health problems associated with the high risk of chronic noncommunicable diseases and obesity.
Revisar sistematicamente as evidências relacionadas aos desfechos nutricionais e cardiometabólicos em crianças nascidas a termo e pequenas para idade gestacional e a relação com o aleitamento materno.
Fonte de dadosDois revisores independentes fizeram buscas nas bases de dados MEDLINE, LILACS, SciELO, EMBASE sem restrições de tempo ou idioma. Foi usada a ferramenta PRISMA sendo incluídos estudos que avaliaram crianças nascidas a termo e pequenas para idade gestacional, amamentadas e com avaliação dos desfechos cardiometabólicos. Foram excluídos estudos com prematuros, aqueles que não trouxessem informação do aleitamento materno, ausência de avaliação das variáveis de desfecho. Também não foram incluídos artigos de revisão, editorial e série de casos.
Síntese dos dadosForam encontrados apenas sete artigos que preencheram os critérios citados acima. Houve uma grande variabilidade na forma de avaliação, assim como na idade dessas crianças. Foi evidenciado que o aleitamento materno promoveu crescimento sem alteração de composição corporal e sem resistência insulínica aumentada nas crianças com aleitamento materno exclusivo, quando comparadas com crianças que receberam fórmula láctea de maior teor calórico, exceto por um artigo que observou aumento de massa gorda nos amamentados exclusivamente.
ConclusãoAleitamento materno parece ser uma forma segura de alimentação para crianças nascidas a termo e pequenas para idade gestacional sem associação com desfechos deletérios em curto prazo. O estímulo ao aleitamento materno nessas populações parece ser um caminho de prevenção aos agravos à saúde associados ao alto risco de doenças crônicas não transmissíveis e à obesidade.
Exclusive breastfeeding (EBF) should be carried out up to the sixth month of life according to the World Health Organization's (WHO)1 feeding recommendations, since, in addition to the nutritional benefit, it is relevant for the prevention of infectious diseases and obesity in childhood, and its protective effect seems to persist into adulthood.2,3
Children born with low birth weight (LBW; birth weight less than 2500g) have a 20-fold higher morbimortality than those born weighing >2500g.4 When LBW is associated with intrauterine growth restriction (IUGR), newborns are defined as small for gestational age (SGA), which seems to be associated with a higher rate of early and late morbimortality from cardiovascular and metabolic diseases in adulthood.5,6 Furthermore, other authors have shown that SGA children who showed greater-than-expected weight gain in the first months of life (“catch up” phenomenon) have an enhanced risk of this outcome.7,8
The benefit of EBF is superior to all other forms of early childhood feeding for the child born with normal weight.9 In turn, for the child born SGA, the protective role of breastfeeding (BF) regarding the future onset of obesity and metabolic syndrome (MS) has not been completely defined. Therefore, the aim of this study was to review the literature on the nutritional and metabolic effects in infants who were born SGA at term and were breastfed.
MethodsThe present study is a systematic review carried out according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) methodology.10 Publications were searched in the MEDLINE, LILACS, SciELO, and Embase databases using as reference technical-scientific terms selected together with a specialist in the field (endocrinologist) and written in the English language, and the search strategy was structured by using Boolean operators specific to the database, namely: low birth weight, LBW, small for gestational age, SGA, intrauterine growth restricted IUGR, breast-feeding, breastfeeding, breastfed, breastmilk, human milk, metabolic syndrome, cholesterol, LDL, HDL, triglycerides, blood lipids, blood pressure, hypertension, systolic blood pressure, diastolic blood pressure, overweight, obesity, body mass index, diabetes mellitus, glucose, glycemia, hyperglycemia, and the Boolean operators AND, OR, and NOT (MEDLINE search procedures). The detailed search resulted in: [(SGA OR small for gestational age OR low birth weight OR IUGR OR intrauterine growth restricted) AND (breastfeeding OR breast-feeding OR breastfed OR breastmilk OR human milk) AND (metabolic syndrome OR cholesterol OR HDL OR LDL OR triglycerides OR blood lipids OR blood pressure OR hypertension OR systolic blood pressure OR diastolic blood pressure OR overweight OR obesity OR body mass index OR diabetes mellitus OR glucose OR hyperglycemia OR glycemia)].
The literature search was performed by two independent researchers. After the identification of the articles by title, the abstracts were read and, for those with potential for inclusion, the full-text was read. In cases of inclusion disagreement, a third researcher made the decision. Relevant secondary references were also included. Articles in duplicate were removed. There was no time or language restriction for article selection.
The quality of the evidence was evaluated according to the criteria proposed by EPHPP (Effective Public Health Practice Project – Quality Assessment Tool for Quantitative Studies).11 These criteria evaluate selection bias, study design, potential confounders, blinding of investigators and participants, data collection methods (if they were valid and reliable), followup losses (exclusion or loss to followup), integrity of the intervention, and appropriate analysis of the research question. The studies were then classified as poor, moderate, or strong quality.
The inclusion criteria were: studies that assessed full-term and SGA children (regardless of the reference curve used) who were breastfed, and some outcome related to MS and/or cardiovascular disease.
Articles that assessed preterm children, those that lacked information on BF or evaluation of outcome variables, non-BF feeding, review articles, editorials, case reports, or series of cases were excluded.
The following outcomes were considered: any laboratory examination related to lipid and glycemic profile; metabolism-related hormones, such as insulin-like growth factor I (IGF-1), adiponectin, leptin and insulin; arterial hypertension; diabetes mellitus (DM); overweight; obesity; body composition and adiposity at any growth period up to adulthood.
According to the rules established by the National Health Council – Ministry of Health Resolution No. 510 of 2016, there is no need for analysis by the Research Ethics Committee.
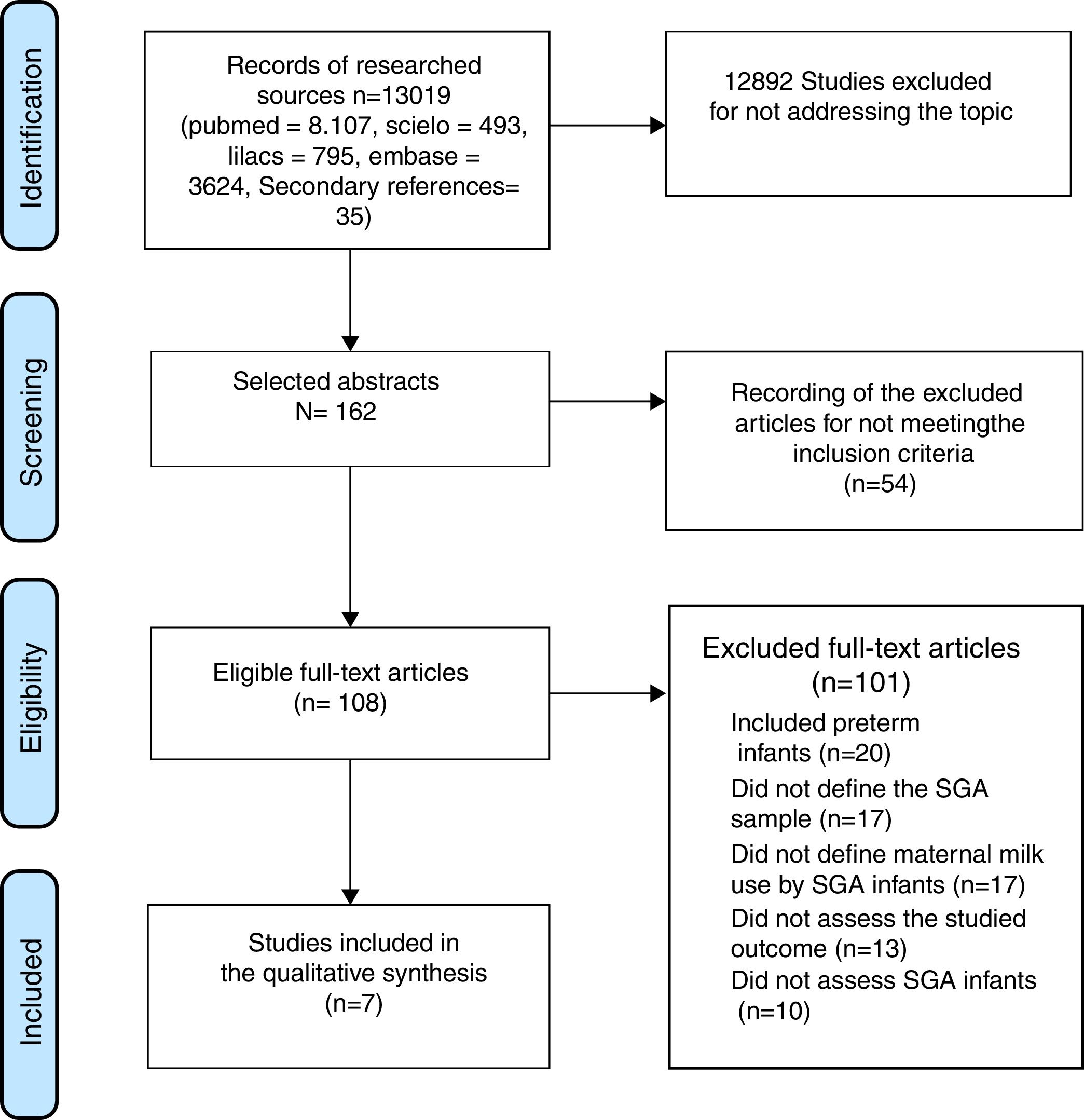
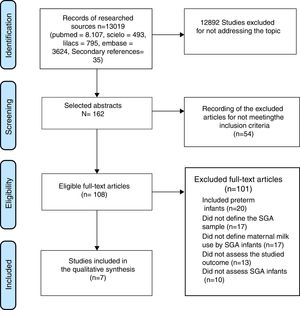
ResultsInitially, 13,019 titles were identified, but the vast majority were excluded because of the generic use of the LBW descriptor, which included patients born weighing less than 1,500g and less than 1,000g (very low birth weight [VLBW] and extremely low birth weight [ELBW]) and preterm infants. The older articles, especially those published before the 1990s, did not discriminate between SGA, preterm, and LBW. Seven articles that met the inclusion criteria were selected (Fig. 1).
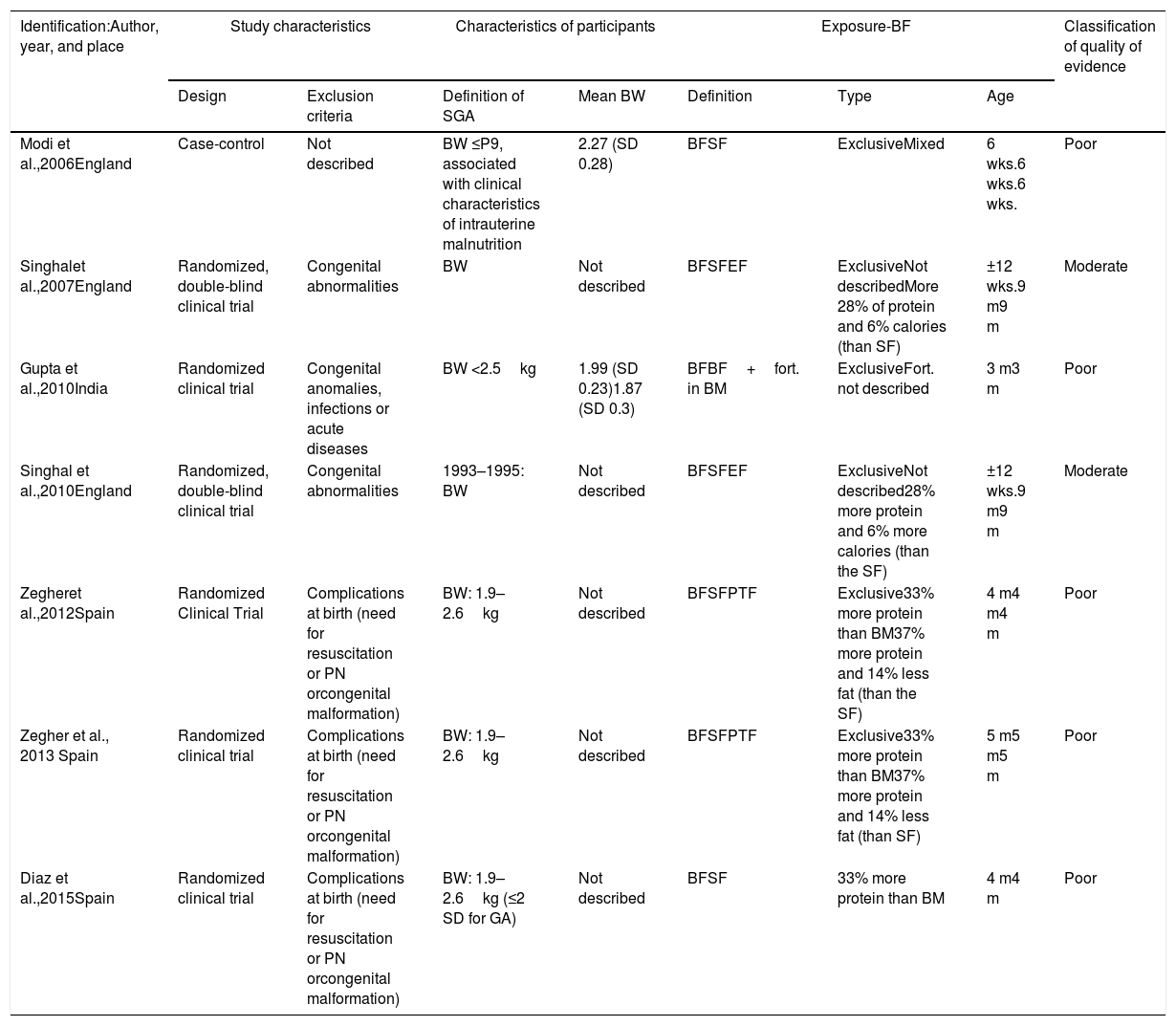
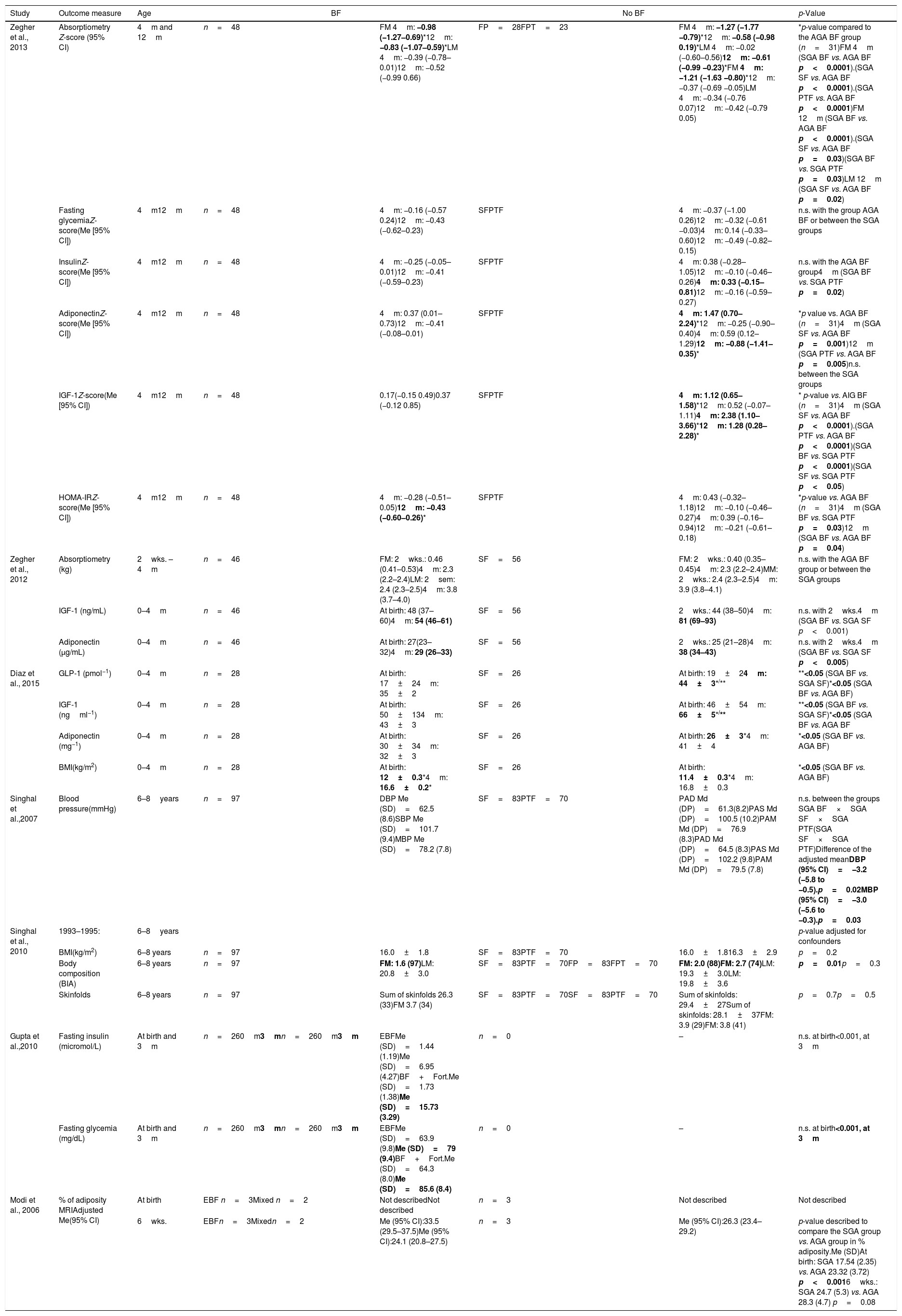
The overall characteristics of the studies included in this review are shown in Table 1 and the main results are shown in Table 2.
General characteristics of the studies included in the systematic review on children born at term and small for gestational age who were breastfed.
| Identification:Author, year, and place | Study characteristics | Characteristics of participants | Exposure-BF | Classification of quality of evidence | ||||
|---|---|---|---|---|---|---|---|---|
| Design | Exclusion criteria | Definition of SGA | Mean BW | Definition | Type | Age | ||
| Modi et al.,2006England | Case-control | Not described | BW ≤P9, associated with clinical characteristics of intrauterine malnutrition | 2.27 (SD 0.28) | BFSF | ExclusiveMixed | 6 wks.6 wks.6 wks. | Poor |
| Singhalet al.,2007England | Randomized, double-blind clinical trial | Congenital abnormalities | BW | Not described | BFSFEF | ExclusiveNot describedMore 28% of protein and 6% calories (than SF) | ±12 wks.9 m9 m | Moderate |
| Gupta et al.,2010India | Randomized clinical trial | Congenital anomalies, infections or acute diseases | BW <2.5kg | 1.99 (SD 0.23)1.87 (SD 0.3) | BFBF+fort. in BM | ExclusiveFort. not described | 3 m3 m | Poor |
| Singhal et al.,2010England | Randomized, double-blind clinical trial | Congenital abnormalities | 1993–1995: BW | Not described | BFSFEF | ExclusiveNot described28% more protein and 6% more calories (than the SF) | ±12 wks.9 m9 m | Moderate |
| Zegheret al.,2012Spain | Randomized Clinical Trial | Complications at birth (need for resuscitation or PN orcongenital malformation) | BW: 1.9–2.6kg | Not described | BFSFPTF | Exclusive33% more protein than BM37% more protein and 14% less fat (than the SF) | 4 m4 m4 m | Poor |
| Zegher et al., 2013 Spain | Randomized clinical trial | Complications at birth (need for resuscitation or PN orcongenital malformation) | BW: 1.9–2.6kg | Not described | BFSFPTF | Exclusive33% more protein than BM37% more protein and 14% less fat (than SF) | 5 m5 m5 m | Poor |
| Diaz et al.,2015Spain | Randomized clinical trial | Complications at birth (need for resuscitation or PN orcongenital malformation) | BW: 1.9–2.6kg (≤2 SD for GA) | Not described | BFSF | 33% more protein than BM | 4 m4 m | Poor |
BF, breastfeeding; SD, standard deviation; StF, starter formula; PTF, preterm formula; GA, gestational age; BM, breast milk; m, months; PN, parenteral nutrition; P, percentile; SGA, small for gestational age; BW, birth weight; wks., weeks.
Main results of the studies included in the systematic review on children born at term and small for gestational age who were breastfed.
| Study | Outcome measure | Age | BF | No BF | p-Value | ||
|---|---|---|---|---|---|---|---|
| Zegher et al., 2013 | Absorptiometry Z-score (95% CI) | 4m and 12m | n=48 | FM 4m: −0.98 (−1.27–0.69)*12m: −0.83 (−1.07–0.59)*LM 4m: −0.39 (−0.78–0.01)12m: −0.52 (−0.99 0.66) | FP=28FPT=23 | FM 4m: −1.27 (−1.77 −0.79)*12m: −0.58 (−0.98 0.19)*LM 4m: −0.02 (−0.60–0.56)12m: −0.61 (−0.99 −0.23)*FM 4m: −1.21 (−1.63 −0.80)*12m: −0.37 (−0.69 −0.05)LM 4m: −0.34 (−0.76 0.07)12m: −0.42 (−0.79 0.05) | *p-value compared to the AGA BF group (n=31)FM 4m (SGA BF vs. AGA BF p<0.0001).(SGA SF vs. AGA BF p<0.0001).(SGA PTF vs. AGA BF p<0.0001)FM 12m (SGA BF vs. AGA BF p<0.0001).(SGA SF vs. AGA BF p=0.03)(SGA BF vs. SGA PTF p=0.03)LM 12m (SGA SF vs. AGA BF p=0.02) |
| Fasting glycemiaZ-score(Me [95% CI]) | 4m12m | n=48 | 4m: −0.16 (−0.57 0.24)12m: −0.43 (−0.62–0.23) | SFPTF | 4m: −0.37 (−1.00 0.26)12m: −0.32 (−0.61 −0.03)4m: 0.14 (−0.33–0.60)12m: −0.49 (−0.82–0.15) | n.s. with the group AGA BF or between the SGA groups | |
| InsulinZ-score(Me [95% CI]) | 4m12m | n=48 | 4m: −0.25 (−0.05–0.01)12m: −0.41 (−0.59–0.23) | SFPTF | 4m: 0.38 (−0.28–1.05)12m: −0.10 (−0.46–0.26)4m: 0.33 (−0.15–0.81)12m: −0.16 (−0.59–0.27) | n.s. with the AGA BF group4m (SGA BF vs. SGA PTF p=0.02) | |
| AdiponectinZ-score(Me [95% CI]) | 4m12m | n=48 | 4m: 0.37 (0.01–0.73)12m: −0.41 (−0.08–0.01) | SFPTF | 4m: 1.47 (0.70–2.24)*12m: −0.25 (−0.90–0.40)4m: 0.59 (0.12–1.29)12m: −0.88 (−1.41–0.35)* | *p value vs. AGA BF (n=31)4m (SGA SF vs. AGA BF p=0.001)12m (SGA PTF vs. AGA BF p=0.005)n.s. between the SGA groups | |
| IGF-1Z-score(Me [95% CI]) | 4m12m | n=48 | 0.17(−0.15 0.49)0.37 (−0.12 0.85) | SFPTF | 4m: 1.12 (0.65–1.58)*12m: 0.52 (−0.07–1.11)4m: 2.38 (1.10–3.66)*12m: 1.28 (0.28–2.28)* | * p-value vs. AIG BF (n=31)4m (SGA SF vs. AGA BF p<0.0001).(SGA PTF vs. AGA BF p<0.0001)(SGA BF vs. SGA PTF p<0.0001)(SGA SF vs. SGA PTF p<0.05) | |
| HOMA-IRZ-score(Me [95% CI]) | 4m12m | n=48 | 4m: −0.28 (−0.51–0.05)12m: −0.43 (−0.60–0.26)* | SFPTF | 4m: 0.43 (−0.32–1.18)12m: −0.10 (−0.46–0.27)4m: 0.39 (−0.16–0.94)12m: −0.21 (−0.61–0.18) | *p-value vs. AGA BF (n=31)4m (SGA BF vs. SGA PTF p=0.03)12m (SGA BF vs. AGA BF p=0.04) | |
| Zegher et al., 2012 | Absorptiometry (kg) | 2wks. – 4m | n=46 | FM: 2wks.: 0.46 (0.41–0.53)4m: 2.3 (2.2–2.4)LM: 2sem: 2.4 (2.3–2.5)4m: 3.8 (3.7–4.0) | SF=56 | FM: 2wks.: 0.40 (0.35–0.45)4m: 2.3 (2.2–2.4)MM: 2wks.: 2.4 (2.3–2.5)4m: 3.9 (3.8–4.1) | n.s. with the AGA BF group or between the SGA groups |
| IGF-1 (ng/mL) | 0–4m | n=46 | At birth: 48 (37–60)4m: 54 (46–61) | SF=56 | 2wks.: 44 (38–50)4m: 81 (69–93) | n.s. with 2wks.4m (SGA BF vs. SGA SF p<0.001) | |
| Adiponectin (μg/mL) | 0–4m | n=46 | At birth: 27(23–32)4m: 29 (26–33) | SF=56 | 2wks.: 25 (21–28)4m: 38 (34–43) | n.s. with 2wks.4m (SGA BF vs. SGA SF p<0.005) | |
| Diaz et al., 2015 | GLP-1 (pmol−1) | 0–4m | n=28 | At birth: 17±24m: 35±2 | SF=26 | At birth: 19±24m: 44±3*/** | **<0.05 (SGA BF vs. SGA SF)*<0.05 (SGA BF vs. AGA BF) |
| IGF-1 (ngml−1) | 0–4m | n=28 | At birth: 50±134m: 43±3 | SF=26 | At birth: 46±54m: 66±5*/** | **<0.05 (SGA BF vs. SGA SF)*<0.05 (SGA BF vs. AGA BF | |
| Adiponectin (mg−1) | 0–4m | n=28 | At birth: 30±34m: 32±3 | SF=26 | At birth: 26±3*4m: 41±4 | *<0.05 (SGA BF vs. AGA BF) | |
| BMI(kg/m2) | 0–4m | n=28 | At birth: 12±0.3*4m: 16.6±0.2* | SF=26 | At birth: 11.4±0.3*4m: 16.8±0.3 | *<0.05 (SGA BF vs. AGA BF) | |
| Singhal et al.,2007 | Blood pressure(mmHg) | 6–8years | n=97 | DBP Me (SD)=62.5 (8.6)SBP Me (SD)=101.7 (9.4)MBP Me (SD)=78.2 (7.8) | SF=83PTF=70 | PAD Md (DP)=61.3(8.2)PAS Md (DP)=100.5 (10.2)PAM Md (DP)=76.9 (8.3)PAD Md (DP)=64.5 (8.3)PAS Md (DP)=102.2 (9.8)PAM Md (DP)=79.5 (7.8) | n.s. between the groups SGA BF×SGA SF×SGA PTF(SGA SF×SGA PTF)Difference of the adjusted meanDBP (95% CI)=−3.2 (−5.8 to −0.5).p=0.02MBP (95% CI)=−3.0 (−5.6 to −0.3).p=0.03 |
| Singhal et al., 2010 | 1993–1995: | 6–8years | p-value adjusted for confounders | ||||
| BMI(kg/m2) | 6–8 years | n=97 | 16.0±1.8 | SF=83PTF=70 | 16.0±1.816.3±2.9 | p=0.2 | |
| Body composition (BIA) | 6–8 years | n=97 | FM: 1.6 (97)LM: 20.8±3.0 | SF=83PTF=70FP=83FPT=70 | FM: 2.0 (88)FM: 2.7 (74)LM: 19.3±3.0LM: 19.8±3.6 | p=0.01p=0.3 | |
| Skinfolds | 6–8 years | n=97 | Sum of skinfolds 26.3 (33)FM 3.7 (34) | SF=83PTF=70SF=83PTF=70 | Sum of skinfolds: 29.4±27Sum of skinfolds: 28.1±37FM: 3.9 (29)FM: 3.8 (41) | p=0.7p=0.5 | |
| Gupta et al.,2010 | Fasting insulin (micromol/L) | At birth and 3m | n=260m3mn=260m3m | EBFMe (SD)=1.44 (1.19)Me (SD)=6.95 (4.27)BF+Fort.Me (SD)=1.73 (1.38)Me (SD)=15.73 (3.29) | n=0 | – | n.s. at birth<0.001, at 3m |
| Fasting glycemia (mg/dL) | At birth and 3m | n=260m3mn=260m3m | EBFMe (SD)=63.9 (9.8)Me (SD)=79 (9.4)BF+Fort.Me (SD)=64.3 (8.0)Me (SD)=85.6 (8.4) | n=0 | – | n.s. at birth<0.001, at 3m | |
| Modi et al., 2006 | % of adiposity MRIAdjusted Me(95% CI) | At birth | EBF n=3Mixed n=2 | Not describedNot described | n=3 | Not described | Not described |
| 6wks. | EBFn=3Mixedn=2 | Me (95% CI):33.5 (29.5–37.5)Me (95% CI):24.1 (20.8–27.5) | n=3 | Me (95% CI):26.3 (23.4–29.2) | p-value described to compare the SGA group vs. AGA group in % adiposity.Me (SD)At birth: SGA 17.54 (2.35) vs. AGA 23.32 (3.72) p<0.0016wks.: SGA 24.7 (5.3) vs. AGA 28.3 (4.7) p=0.08 | ||
AGA, adequate for gestational age; BF, breastfeeding; EBF, exclusive breastfeeding; BIA, bioimpedance analysis; SD, standard deviation; Fort, food fortifying agent; SF, standard formula; PTF, preterm formula; GLP-1, glucagon-like peptide-1; HOMA-IR, homeostatic model assessment – insulin resistance; CI, confidence interval; BMI, body mass index; IGF-1, insulin growth factor 1; m, months; Me, mean; FM, fat mass; LM, lean mass; n.s., non-significant; DBP, diastolic blood pressure; MBP, mean blood pressure; SBP, systolic blood pressure; SGA, small for gestational age; MRI, magnetic resonance imaging.
Measurement and p-values where the differences between groups were statistically significant are shown in bold.
Of the seven articles included, only two were classified as having moderate quality of evidence, whereas the others were considered poor. One of the articles, in addition to the poor quality, had a small sample size (eight patients studied).
In the article by Singhal et al., published in 2010, only the results related to study 1 were considered, since only this part of the study took into account children born SGA and had a breastfed control group.
Three of the articles included in this review were from the same cohort, carried out by Zegher et al. during 2012 and 2013 and Diaz et al. during 2015 in Spain, published at different times during the months of followup. In the 2012 study, it was observed that SGA children, regardless of the type of diet offered, had similar gains in weight, height, and adiposity. When compared to the breastfed AGA group, they found that SGA children had gained more lean mass and less fat mass at 4 months of age. At the laboratory evaluation at birth, SGA children had lower levels of IGF-1 and adiponectin than the AGA children, but at 4 months of age, IGF-1 levels in SGA children receiving fortified formula for preterm infants and adiponectin in the SGA group receiving standard formula were higher than the values in SGA and AGA children that were breastfed.12 The same pattern was found in the study by Diaz et al. in 2015 regarding GLP-1 levels, a peptide that has a potential as an appetite modulator, which showed higher levels and increments in the SGA group with formula than in those who were breastfed (SGA and AGA).13 The other study of the same cohort that followed children from 4 to 12 months of age found that SGAs receiving BF maintained hypoadiposity (more lean mass than fat mass), with normal levels of IGF-1 and adiponectin. The SGA children that received fortified formula for preterm infants showed fat mass “catch up,” persistently high levels of IGF-1 and decreased adiponectin levels. The group that received standard formula showed an intermediate development: less intense fat mass “catch up” than those receiving fortified formula and laboratory tests similar to those of SGA children who were breastfed.14
In 2007, Singhal et al. published a randomized clinical trial from 1993 to 1995 and compared standard formula for full-term infants, fortified formula for preterm infants, or breastfeeding in SGA individuals, with the formulas being maintained until the ninth month. The authors observed that the rate of weight gain was directly related to higher blood pressure levels at 6–8 years of age, regardless of the type of diet offered, and including the breastfed group, but it was more frequent in the group that received fortified formula. The authors recommended that one should not seek rapid weight gain with a greater caloric supply in these children and, for this purpose, breastfeeding should be stimulated, stating that the latter promoted slower weight and height gain.15 Subsequently, these same authors published the results of the 1995 clinical trial with another one started 10 years later.16 In this second study, SGA children were classified differently (born below the 20th percentile) and it also compared the standard formula for full-term infants or fortified formula used until the 6th month of life; however, only the first study had a breastfed SGA control group and, for this reason, the results of this second phase were not presented. The children who received fortified formula showed faster weight gain in the first months of life, which was related to increased body fat later in life. The unfavorable findings of the first study interrupted the recruitment for the second one, not justifying the increase in caloric supply in these children.
In 2010, Gupta et al. carried out a randomized clinical trial in India with children born at term (>38 weeks) and weighing <2500g. The authors investigated the effects of early feeding on increased insulin resistance. The subjects were grouped as: group 1, which received EBF for 3 months, and group 2, which received BF and human milk fortifier for the same period. There was no difference between the groups in the first 15 days of life regarding the anthropometric measurements and the glycemia and insulin levels. At the end of the 3rd month, however, these parameters were re-evaluated and the group that received the highest caloric intake through breast milk fortifier developed significantly higher weight and height than the exclusively breastfed group, associated with increased glucose and insulin levels. The authors suggested that those children who had intrauterine growth restriction, after receiving hypercaloric feeding during the first months of life, showed rapid weight gain and developed insulin resistance.17
The study by Modi et al. in 2006 showed an increase in adiposity related to BF in children born with IUGR. The authors included normal full-term (FT) AGA newborns compared to FT infants with IUGR and evaluated the association between diet (EBF×mixed BF×standard formula) with adiposity at 6 weeks of age. They used measurements of adiposity at birth by magnetic resonance imaging (MRI) as a comparison. The authors found that, at birth, neonates with IUGR had lower anthropometric measures (weight, height, and head circumference) and less adipose tissue. However, at 6 weeks, although smaller and leaner, differences in head circumference and adiposity measures were no longer evident when compared to those born to AGA. This increase in adiposity was related to fat mass and, after the regression analysis, they observed that it was related to an increase in linear growth and not to weight gain, especially in those with IUGR and receiving EBF. As BF has been historically related to better metabolic outcome and lower adiposity, the authors of this study questioned whether the small number of patients in the analysis might have influenced the results, since only three children with IUGR were exclusively breastfed during the period.18
DiscussionExclusive breastfeeding up to the 6th month of life is the ideal food for the full-term child, regardless of the birth weight.9 The present review aimed to evaluate whether children born SGA at term and who received BF as the main source of nutrition in the first months of life would benefit from reduced adiposity in childhood and future metabolic disorders.
It remains unclear which factors during childhood would be determinants of risk for cardiovascular diseases in adult life. While historical cohorts point to the influence of LBW,5 other authors believe that the “catch up” phenomenon or high birth weight would be the major etiological factors of obesity, cardiovascular disease, and metabolic syndrome in the future.19–22 However, little emphasis has been placed on the role of nutrition in the early years of the child's life.
The present review identified articles that demonstrated that BF in SGA children born at term can improve the laboratory outcome related to insulin resistance and adiposity measurements. It was also observed that, when breastfed, these children were able to catch-up growth without nutritional impairment, reinforcing that the BF can be the reference food for healthy growth in children born SGA in the first years of life, with effects persisting until the preschool age.
These findings are in disagreement with the hypothesis developed by Barker's group, based on a study of individuals born between 1934 and 1944, according to which the change in metabolism caused by intrauterine growth restriction would determine the risk of obesity and cardiovascular disease up to adult life.23 This was a retrospective study and used secondary data. It found that more than 90% of the population was breastfed, and the association between the lowest birth weight and weight at 1 year of age was linked to higher mortality from ischemic heart disease after 50 years of age. As limitations, this study did not evaluate important risk factors associated with cardiovascular morbimortality, such as smoking, sedentary lifestyle, and other dietary and behavioral influences over the years.23
Three other studies that included preterm infants in the sample were also able to demonstrate the beneficial effect of BF: Rodriguez-Lopes et al.24 evaluated a sample of infants born at term and preterm with IUGR and found subtle signs of cardiovascular alteration (lower left ventricular wall sphericity index, increased carotid wall thickness, and increased blood pressure levels), when compared to infants born AGA. In those who received BF at over 6 months of age, these alterations regressed, probably due to the protective effect of the high content of long chain unsaturated fatty acids contained in breast milk. Similarly, Duran et al.25 in a sample of children, some with LBW and some preterm, also found that for each month of BF there was a reduction of 0.15 in body mass index (BMI) in 1-year-old children and that BMI showed a direct association with diastolic blood pressure measurements at this age, both in the total sample and in the subgroup that was born SGA. The third study, a cohort of adolescents in Spain, emphasized that BF for more than 3 months in children born with LBW contributed to a lower accumulation of abdominal fat.26 These findings suggest that not only the intrauterine period, but also postnatal diet and growth interfere with future health outcomes, and that promoting BF in a population at high risk for adiposity may bring benefits.
Observational and cross-sectional cohort studies26–30 found an association between birth weight, overweight/obesity development, and future cardiovascular disease, also emphasizing the protective role of breastfeeding in children with LBW. However, they did not clarify the percentage of subjects in the subgroup with LBW who received BF. In order to understand the association between BF and its direct benefit for children born SGA or with LBW, it was necessary to perform this review. The same occurred in other studies that investigated the association between birth weight and postnatal weight gain velocity (catchup) and found different results regarding adiposity and breastfeeding,31–33 but did not define the group submitted to BF. In the present review, the selected articles have filled this gap.
The study by Singhal et al.16 showed that the increased caloric supply in SGA infants, especially protein, not originated from breastfeeding, led to a rapid early growth with fat mass accumulation. Perhaps because of the evidence that the increase in the caloric supply through artificial formulas is deleterious to children born SGA, recent studies aimed to investigate the effect of dietary interventions have been discouraged. In that study, as well as in other studies mentioned in the present review, except the one by Modi et al.18 when the caloric supply to children born SGA was carried out through BF, there were beneficial effects.
Most likely, intrinsic components in breast milk might influence the growth of at-risk children, including non-nutritional components.34 Associated to that fact, breast milk adipokines (leptin and adiponectin) may lead to a better appetite regulation, helping to control weight gain in the future, improve insulin sensitivity, and increasing fatty acid metabolism.35 Regarding children born SGA, it seems that BF is associated with a healthier weight gain, reducing the impact caused by intrauterine malnutrition.
It should be emphasized that, based on the data obtained in the present review, the positive role of breastfeeding for infants born SGA was observed. These results, however, should be interpreted with caution, since the different studies did not have a uniform design, and there was a diverse SGA classification. Additionally, only two studies had a moderate quality score, while the others were considered poor. The studies showed variability regarding the age at which the children were evaluated, and the type of evaluation related to the effects of BF. Multiple classifications of BF and time of weaning were an important limitation for the analysis of the studies. The lack of standardization and the small number of study subjects also made it difficult to interpret and generalize the results, and made it impossible to perform metaanalysis. However, the homogeneity of the final outcome of the articles included in this review suggests the benefit of BF in the prevention of obesity and metabolic and/or cardiovascular diseases in the future of children born SGA.
The effectiveness of the prenatal follow-up to reduce the frequency of LBW (related to prematurity or IUGR) at birth is a universal recommendation, especially in developing countries.36 This review brings the additional recommendation that BF stimulation in these populations seems to prevent the health problems associated with the high risk of chronic noncommunicable disease and obesity.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Santiago AC, Cunha LP, Vieira NS, Moreira LM, Oliveira PR, Lyra PP, et al. Breastfeeding in children born small for gestational age and future nutritional and metabolic outcomes: a systematic review. J Pediatr (Rio J). 2019;95:264–74.
The study is linked for registration purposes in the Index Medicus/MEDLINE database to Universidade Federal da Bahia, Salvador, BA, Brazil.