To describe the main findings of studies of behavioral and neural correlates regarding the development of facial emotion processing during the first year of life in typically developing infants and infants of depressed and/or anxious mothers.
SourcesComprehensive, non-systematic review of the literature on studies about individual differences in facial emotion processing by newborns and infants over the first year of life.
Summary of the findingsMaternal stress related to depression and anxiety has been associated to atypical emotional processing and attentional behaviors in the offspring. Recent neurophysiological studies using electroencephalogram and event-related potentials have begun to shed light on the possible mechanisms underlying such behaviors.
ConclusionsInfants of depressed and/or anxious mothers have increased risk for several adverse outcomes across the lifespan. Further neurobehavioral investigations and the promotion of clinical and developmental research integration might eventually contribute to refining screening tools, improving treatment, and enabling primary prevention interventions for children at risk.
Descrever os principais achados de estudos de correlação entre o comportamento e as bases neurais em relação ao processamento de emoções faciais durante o primeiro ano de vida de lactentes com desenvolvimento típico e lactentes de mães deprimidas e/ou ansiosas.
FontesAnálise abrangente e não sistemática da literatura de estudos sobre diferenças individuais no processamento de emoções faciais de neonatos e lactentes ao longo do primeiro ano de vida.
Resumo dos achadosO estresse materno relacionado à depressão e ansiedade tem sido associado a alterações no processamento emocional e na alocação da atenção da prole. Estudos neurofisiológicos recentes utilizando electroencefalograma e potenciais relacionados a eventos começam a esclarecer os possíveis mecanismos inerentes a esses comportamentos.
ConclusõesLactentes filhos de mães deprimidas e/ou ansiosas têm maior risco de problemas de saúde física e mental durante toda vida. O avanço de estudos neurocomportamentais e a promoção de integração entre a pesquisa clínica e de desenvolvimento poderão contribuir para refinar as ferramentas de triagem, melhorar o tratamento e permitir intervenções de prevenção primária para crianças em risco.
The ability to recognize and understand facial expressions of emotion is a fundamental skill in daily interactions with others, and plays a particularly important role early in life, before the onset of language.1 Face recognition is one of the most salient cues for social interaction and affective communication. Facial expression recognition develops gradually during infancy and childhood, and appears to continue to develop until early adulthood.2 During the first year of life, however, the development of visual orientation and the discrimination of different emotions progresses rapidly.3 Over the past several decades, behavioral studies4–6 have used different measures of visual preference to infer aspects of recognition of emotional faces. More recently, new methods to elucidate distinct correlates of brain activation have provided significant contribution to the field. Two of the most used methods, known as electroencephalogram (EEG) and event-related potentials (ERP), are reviewed here.
Evidence has become available that stress exposure during pregnancy and the postnatal period leads to several long lasting detrimental outcomes in the offspring, including behavior and cognitive problems and neurodevelopmental delay.7 Studies on maternal negative affective states, including both depression and anxiety, indicate a detrimental effect on the child's health and development, increasing the risk for a wide range of disorders such as low birth weight and preterm birth, cognitive and motor developmental delay, achievement deficits, and psychiatric disorders.8 Infants of depressed and anxious mothers have increased vulnerability to cognitive and emotional problems throughout their lifespan.7,8
Psychological stress, depression, and anxiety are closely linked and often coexist.7 Approximately 10–20% of women will exhibit symptoms of depression during pregnancy and/or the postpartum period.7 Anxiety disorders in the perinatal period have received more scientific attention only recently and their prevalence is still unclear, yet estimates range as high as 30%.9 The outcomes of anxiety and depression are often studied together, as the symptoms frequently overlap, and their coexistence is a marker of severity.9,10
The mechanisms between maternal negative affective states and the infant outcomes are studied both in animal and human clinical research. During pregnancy, maternal stress induces the dysregulation of the hypothalamic-pituitary-adrenocortical (HPA) system, elevating the cortisol levels and inducing sympathetic activation with release of catecholamines.7,9 The latter is associated to increased uterine artery resistance, reducing the blood flow to the fetus, with restricted inflow of oxygen and nutrients.10,11
The higher levels of maternal cortisol adversely affect fetal brain development, possibly due to epigenetic dysregulation through alterations in synaptogenesis and neurotransmitter functions.11,12 There is evidence of disruption of the fetal HPA system, with adverse physiological and biochemical effects on the fetus and newborn10 that can persist throughout infancy, resulting in altered infant perception and behavior.
In the postnatal period, maternal anxiety and depression are related to less sensitive and inconsistent care when interacting with infants, providing suboptimal levels of general stimulation, and disrupting the mother–child relationship and the formation of attachments.8,13 Accumulating evidence indicates that the emotional environment of the infants’ daily experiences influences their developmental trajectory of facial recognition.14 Typically, the mother is the most present person in an infant's life, and the mother's facial expressions are the most prevalent in their experience.14 Mothers with depression and anxiety smile less, display more flat affect and negative facial expressions, and interact with infants in a withdrawn and muted style.14,15 As a result, infants of depressed and/or anxious mothers have systematically atypical social experiences compared to infants of healthy mothers.14,16 Understanding the possible mechanisms by which maternal stress related to depression and anxiety affects an infant's development is a key to further developing better intervention strategies and prevention programs.
This review examines the findings of studies about maternal depression and anxiety on face recognition by the infant, both regarding behavior and neurophysiology. It begins with a brief review of typically developing infants in order to set the stage for the discussion that follows, focused on infants of depressed and/or anxious mothers.
Behavioral studiesThe majority of early emotional behavioral studies measure looking time or visual preference. Visual fixation gradually decreases to a repeatedly presented stimulus, a phenomenon referred to as habituation (or familiarization); presentation of a new stimulus leads to recovery of looking if the infants can discriminate the old from the new stimulus (dishabituation).3 This measure can be used to examine the ability to discriminate different visual stimuli, such as one face from another or one object from another, or in the current context, one facial expression from another. Another method to measure visual preference is the visual paired comparison procedure, in which looking time and duration of the first visual fixation are measured comparing two expressions seen at the same time.3
Emotional information can also be inferred from multisensory modalities presented simultaneously, such as facial and vocal stimuli. Multimodal studies investigate how different sensory stimuli may influence the processing and perception of each other.5,17 For example, studies analyzing congruent and incongruent face–voice pairs (i.e., same or different emotion presented by the face–voice) attempt to elucidate how emotional information is integrated.5
Furthermore, emotional responses are measured observing infants’ specific behavioral reactions, such as facial expressions, vocalization, imitation, or body movements. One commonly used paradigm is the still-face, in which the mother (or the experimenter) is instructed to show flat affect, mimicking emotional unavailability. Infants typically respond with distress, manifested by less motor activity, frowning, gaze aversion and crying.13,18,19
Neural correlates - Electroencephalogram (EEG)The EEG is a measure of electrophysiological brain activity that represents synchronized activation of large populations of cortical pyramidal neurons firing together. The synchronization of electrical activity generates different continuous frequencies of oscillation, measurable by electrodes placed at the scalp. EEG is a non-invasive method that can be used in infant-friendly environments and has an excellent temporal resolution.20 The study field of emotional development has especially explored the pattern of frontal EEG power in the alpha frequency.20,21 Alpha frequency appears early in life, matures rapidly over the first few years, and thereafter remains relatively stable.22 Alpha power is inversely related to brain activity, confirmed with hemodynamic and metabolic measures (i.e., negatively correlated with cerebral perfusion in functional magnetic resonance imaging (fMRI) and with cerebral glucose metabolism using positron emission tomography (PET)).16,21 Therefore, EEG alpha power is used as an inverse indicator of regional cortical activation. The EEG frontal asymmetry (FA) is a measure computing the difference between the scores of alpha power comparing frontal right and left areas.21,23
A large body of empirical work measuring FA relates different patterns of activation to differentially specialized types of emotions.20,21 Greater relative left FA is associated with approach behaviors (such as joy, anger, and surgency) and with the expression of positive affect. In turn, greater relative right FA is associated with avoidance and withdrawal behaviors, as well as the expression of certain negative emotions, such as fear and sadness. The studies16,21–24 of FA are related to both trait and state measures, considering individual differences in affective style and emotional disorders, and acute affective response, respectively.
Neural correlates - Event-related potentials (ERPs)ERPs are the most common method used in infancy to investigate the neural correlates of a variety of perceptual and cognitive functions. ERPs are transient changes in brain activity that occur in response to a discrete event, extracted from the EEG recording. Electrical brain activity is measured during the presentation of repeated stimuli, revealing reliable patterns according to each stimuli category. It is successfully used to investigate perception discrimination, emotion recognition, and memory in infants and adults.25
Studies in infants have identified several components involved in visual human face processing: P1 (positive deflection that peaks around 120ms after stimulus onset), N290 (negative deflection at 290ms post stimulus), P400 (positive deflection at 390–450ms for infants between 3 and 12 months of age), Nc (Negative central deflection peaking around 400ms after stimulus), and positive slow wave (PSW, positive deflection beginning around 800ms after stimulus).25,26 The precise meaning of each component and the developmental trajectory remain to be clarified both in children and in adults, although a number of recent studies have begun to shed light on this subject. Specifically, consistent evidence relates similar face-sensitive processing for both the N290 and the P400 to the N170, a component reliably studied in face processing in adults.25 Additionally, the Nc is considered an index of attention and orienting to salient stimuli in infants, stimuli that recruit more attention appear to enhance the amplitude of the Nc.27
The electrophysiological processes underlying multimodal sensory integration in emotion began recently to be examined throughout development. Auditory ERP components can be explored in paradigms using simultaneous face–voice stimuli.5 Some authors have shown that the previously described Nc and a positive component (Pc, a component with similar characteristics of the PSW, possibly equivalent to it) are also sensitive in crossmodal face–voice stimuli. The enhanced amplitude of the Nc is related to attention orienting to unexpected/unfamiliar stimuli, while the Pc evinces a larger amplitude to familiar stimuli.17 Other researchers refer to infants’ responses to auditory stimuli as the P150-N250-P350-N450 ERP complex, and consider the N450 equivalent to the Nc.28 These infant components are believed to be precursors of children's and adults’ components (P1, N1, N2, P2, P3a, and N4) and can already be observed at birth.28
Typically developing infantsStudies with newborns reveal that infants already look longer and preferentially orient to face-like stimuli several hours after birth,29 suggesting they have some ability to orient to the most salient social stimuli in their environment: faces. The developmental process is defined as “experience expectant”: the innate neural architecture has the potential to become specialized for face processing, but it needs to be primed through experience, allowing the face-processing pathway to mature.26
A few studies30–32 have shown that newborns already react distinctly to different facial expressions. Field et al.30,31 conducted a series of studies with newborns, both term and preterm, using dishabituation procedure and behavior observation. The authors reported that newborns were able to discriminate and imitate happy, surprise, and sad expressions (although there was no control group in this study). In a more recent dishabituation study, term newborns showed increased looking time to happy compared to fearful faces presented at the same time, and no difference between neutral and fearful categories.32
Even though there is evidence that newborns might differentiate some facial expressions, it is not until 3–4 months of age that infants can reliably distinguish among happy compared to some other emotional expressions.5 Infants at 3 months of age can discriminate among happy and surprised faces,5 and between happy and sad faces of their own mothers or a stranger.6 In another experiment,4 3-month-olds discriminated between happy and neutral faces and within the positive emotional category (i.e., different degrees of happy), demonstrating increasing positive visual preference with the intensity of the smile rising, peaking with maximally toothy smiles. In this study, maternal style to the infants’ perceptual sensitivity was categorized. Mothers who actively encouraged their infants to attend to them more often had infants who detected facial expressions of smiling more readily, a possible reflection of the effects of early experience with the mother's interaction styles.4
Accordingly, happy faces appear to be the first to be discriminated in infants comparing to all other facial expressions.29 The infant's early preference for positive emotions is believed to be related to the necessity of bonding at this attachment formation stage.29 As the infant pays more attention, the caregiver smiles and the infant imitates it back, promoting a positive environment and strengthening their relationship, crucial to the infant's survival.4,29
The studies on infants after the age of 7 months present more consistent data of the infant's ability to categorize other expressions than happiness.5 In fact, at some point between 5 and 7 months of age, infants develop a preference for fearful faces over other emotions.1 Seven-month-old infants look longer to fearful than to neutral or happy faces, and are less likely to disengage attention from fearful faces.1,33 This pattern is typically seen in adults, possibly to prioritize the identification of potential environmental threats.1 As infants begin to crawl, and their locomotion ability improves this response, thus may reflect an adaptive increase in vigilance in response to cues of threat in the environment.1,33
In a multimodal face–voice experiment, 5-month-old infants reliably detected emotional vocal changes, but only if there was a simultaneous presentation of faces, suggesting that facial cues might facilitate infants’ perception of emotional voice tones.34 In another study, 7-month-old infants recognized face–voice common affect, displaying preference for face and voice emotionally congruent matching stimuli rather than incongruent ones, even when voice was played out of synchrony with the face.5
Neural correlatesStable patterns of FA emerge early in life and are related to individual differences in emotional trait dispositions, such as emotion regulation and reactivity.16,20,21 Infants with more difficult temperaments (i.e., highly reactive, fearful, and inhibited) show greater relative right FA.7,10 FA is also observed in acute affective response, indicating a current emotional state. Fox and Davidson35 conducted a series of studies examining FA during a variety of elicitors producing positive or negative emotions. Newborns were tested with water, sucrose, and citric acid solutions while concomitant EEG was recorded. The solutions elicited facial expressions that the experimenters coded as interest and disgust, which were associated to greater left FA in response to a pleasant taste (sucrose) compared to unpleasant (citric acid) or neutral tastes.35
Typically developing 10-month-old infants watched videotaped segments of a female model displaying happy or sad facial expressions. The infants showed greater relative left FA when observing the happy expressions.36 In another study,23 the same authors documented asymmetrical EEG activation in the frontal cortex when 10-month-old infants were observed while exhibiting specific behaviors. Infants showed greater left FA when expressing approach behaviors like reaching with hands for their mother and eliciting facial expressions of joy accompanied by positive vocalizations. When the same infants displayed behaviors such as gaze aversion and distress (i.e., active withdrawal behavior), there was greater relative right FA.23 In a similar posterior study, 6-month-old infants who demonstrated fear and sadness (while a stranger was approaching) had greater right FA.37
In the first study in infants using ERPs to analyze differences in response to facial emotional states, Nelson and De Haan27 reported that 7-month-old infants evinced a larger Nc when seeing fearful instead of happy faces, and showed no differences when tested only on negative emotions (angry and fear). The authors also reported variations on the amplitude of two positive components, an early and a later positive component, both of greater amplitude to happy rather than to fearful faces. Additional studies corroborate with the finding of enhanced Nc amplitude and attentional response to emotional face stimuli.1,23,33
In a multimodal processing study, 7-month-old infants demonstrated a larger Nc for emotionally incongruent face–voice pairs of happy and angry stimuli, whereas congruent stimuli elicited larger amplitude for the Pc.17 The pattern of an attenuated Nc and a larger Pc was related to the recognition of the congruent pairing.17 Recently, 9-month-old infants revealed modified auditory ERP components (larger positivity on P150 and P350 and smaller negativity in N250 and N450) for either happy or fearful vocalizations when preceded by visual exposition to fearful faces.38 Both P150 and P350 are related to orienting attention; therefore, the authors concluded that fearful faces enhanced attentional levels, modulating ERP responses.38
Infants of depressed and anxious mothersMaternal depression and anxiety are implicated in atypical behaviors in infants since birth.9 Infants of depressed and anxious mothers are believed to have higher arousal and less attentiveness,12 showing less orientation to facial expressions and face–voice pairs in experimental conditions and in live face–voice interactions.15 Studies of behavior and neural correlates are beginning to improve insights into the mechanisms underlying the presumably slower sensory processing and delayed attention in these infants.
Most of the studies on maternal negative affective states are based on symptoms scales rather than a confirmed diagnosis of maternal depression and anxiety. Although self-report scales do not provide a clinical diagnosis, they have been correlated with confirmed diagnoses on clinical evaluations.8,9,22 It is particularly noteworthy to emphasize that the use of depression symptoms scales has been shown to invariably assess a wide range of anxiety symptoms, as well as other negative affective states such as anger and irritability.10,11 Therefore, although the majority of studies reviewed here rely on depression symptoms scales, it is believed that the related outcomes may be secondary to maternal anxiety aspects as well.8–10
Studies on newborns indicate that infants of depressed mothers (IDMs) orient less to faces and voices as early as during the first hours of life. Hernandez-Reif et al.39 tested full-term newborns of depressed and non-depressed mothers for visual preference and habituation to the mother's face–voice, comparing to a female stranger. IDMs required one-third more trials and almost twice as long as the infants of nondepressed mothers (INDMs) to habituate to their mother's face–voice pairing. In the post-test visual preference phase, IDMs failed to discriminate their mothers from a stranger. In a subsequent experiment,40 a group of mothers were evaluated longitudinally for continuity of depressive symptoms pre- and postnatally, including comorbid anxiety. Their 3-month-old infants were exposed to video clips of female models with face and voice stimuli for happy and sad conditions. As earlier reported in newborns, IDMs required longer time to habituate to faces, particularly to happy facial expressions. Unexpectedly, IDMs were able to discriminate sad from happy expressions but only if they were first habituated to sad, thereby indicating that they may not perceive sad expressions as a novelty.40
Using a still-face procedure, 3-month-old IDMs exhibited less distress and fewer negative expressions comparing to the typical response of INDMs, possibly related to being more accustomed to a less expressive environment characterized by their mothers’ relatively flat affect and less interactive behaviors.18,19 Beyond that, IDMs had a less interactive behavior (i.e., fewer positive and negative behaviors) during the recorded spontaneous mother-infant interactions, when mothers were instructed to engage with their infant in play, as they would usually do at home.19 In a study13 that added a tactile component to the still-face procedure, mothers were asked to maintain a neutral face while touching the infant. Three-month-old IDMs showed more positive affect, manifesting more smiles and vocalizations than infants in the still-face control group without touching. The authors suggest that providing touch stimulation can increase infants’ attention and positive affect, thus improving the interactions of depressed mothers and their infants.13
Five-month-old infants of mothers with a confirmed diagnosis of depression were successfully habituated to neutral or smiling faces, but they later failed to discriminate between the facial expressions.14 Striano et al.41 investigated 6-month-old IDMs and INDMs and found that all infants were able to discriminate neutral from progressively higher intensities of smiling and frowning faces. IDMs, however, presented a looking preference for all smiling faces, and a greater preferential looking to high intensity smiling and frowning expressions, a pattern not observed in INDMs.
Hence, there is evidence that IDMs show less interest in faces, orient poorly to synchronized visual and vocal stimuli, and have diminished sensitivity to changes in facial expressions from birth and throughout the first months of life.15 The abnormalities are speculated to be related to deficits in attentiveness, as well as altered perceptual skills, perhaps secondary to atypical visual and/or auditory sensory processing.14,15
Neural correlatesThere is consistent evidence that IDMs show greater relative right FA than do infants whose mothers are not depressed. This pattern is reported from newborns studies, remaining stable throughout infancy up to childhood.16,20 The right FA bias is also exhibited by depressed adults, which remains stable even after a remission of the depressive symptoms.22 Relative right FA is also associated with anxiety.11
During face-to-face interactions, 3-month-old IDMs compared to INDMs were less responsive to facial expressions, looked longer at sad faces, and displayed less positive and more often negative faces themselves.42 Another group of 3-month-old watched videos of a female model displaying happy and sad facial and vocal expressions. The INDMs exhibited greater relative right FA when viewing sad compared to happy face–voice stimuli. No differences on FA were found for IDMs, possibly because the EEG data were analyzed during the whole experiment, rather than accounting for only the periods when the infants were actually looking at the videos.43 In a subsequent study, the same researchers analyzed 3–6-month-old IDMs’ and INDMs’ EEG responses to mothers’ and strangers’ happy, surprised, and sad facial expressions. This time, the researchers only computed the EEG data for the periods when the infants were attending to facial expressions. Both groups of infants showed greater right FA during their mothers’ and strangers’ sad vs. happy expressions; however, IDMs had significantly greater FA compared to INDMs throughout the different expressions of both the mothers and strangers. IDMs were less interested in facial expressions, showed less positive and more negative affect, and evinced increased salivary cortisol levels after the experiment.24
Diego et al.44 further investigated infants of mothers with two different styles of depressive behavior, intrusive and withdrawn. Accordingly, the interaction style and the biochemical profiles differ between the two styles. The withdrawn mothers tended to have more flat affect, less frequent vocalizing as well as touching, and lower dopamine levels. Their infants also showed lower dopamine levels and displayed greater relative right FA. Intrusive mothers showed rough physical contact and quick and loud verbal behavior when interacting with their infants. In the study, 3-month-old infants observed their own mother and a stranger, in happy, surprised, and sad expressions. Infants of intrusive mothers looked longer to surprise and sad expressions compared to happy ones performed by a stranger, and displayed a concomitant greater relative right FA activity. These infants also had an increased salivary cortisol, possibly reflecting a higher response to the stressful stimulus. The nature of the different maternal depressive styles and how they affect the infants’ physiology and behavior have not yet been fully understood and require further research.
As previously reviewed, ERP provide an excellent tool to temporarily correlate behavior and neural response and is easily administered throughout development.25 A growing number of studies have been performed in children and adults, expanding the research on neural vulnerability markers for psychopathological disorders, including depression and anxiety.25 In infancy, a few studies on auditory ERP and maternal affective disorders have begun to demonstrate neural processing alterations, providing insights into the underlying developmental pathways that remain to be better clarified.
In a recent study using ERP and multimodal processing, Otte et al.12 analyzed 9-month-old infants exposed to maternal anxiety during pregnancy (10% of the mothers also reported previous treatment for depression). Infants were presented with happy and fearful facial and vocal stimuli. Infants prenatally exposed to higher levels of anxiety exhibited significantly larger P350 amplitudes and a trend for larger P150 amplitudes after fearful vocalizations, regardless of the preceding visual emotion type, potentially related to an increased attention to fearful vocal stimuli. The findings corroborate studies in children and adults that anxiety symptoms heightened the sensitivity to threat-related information.12
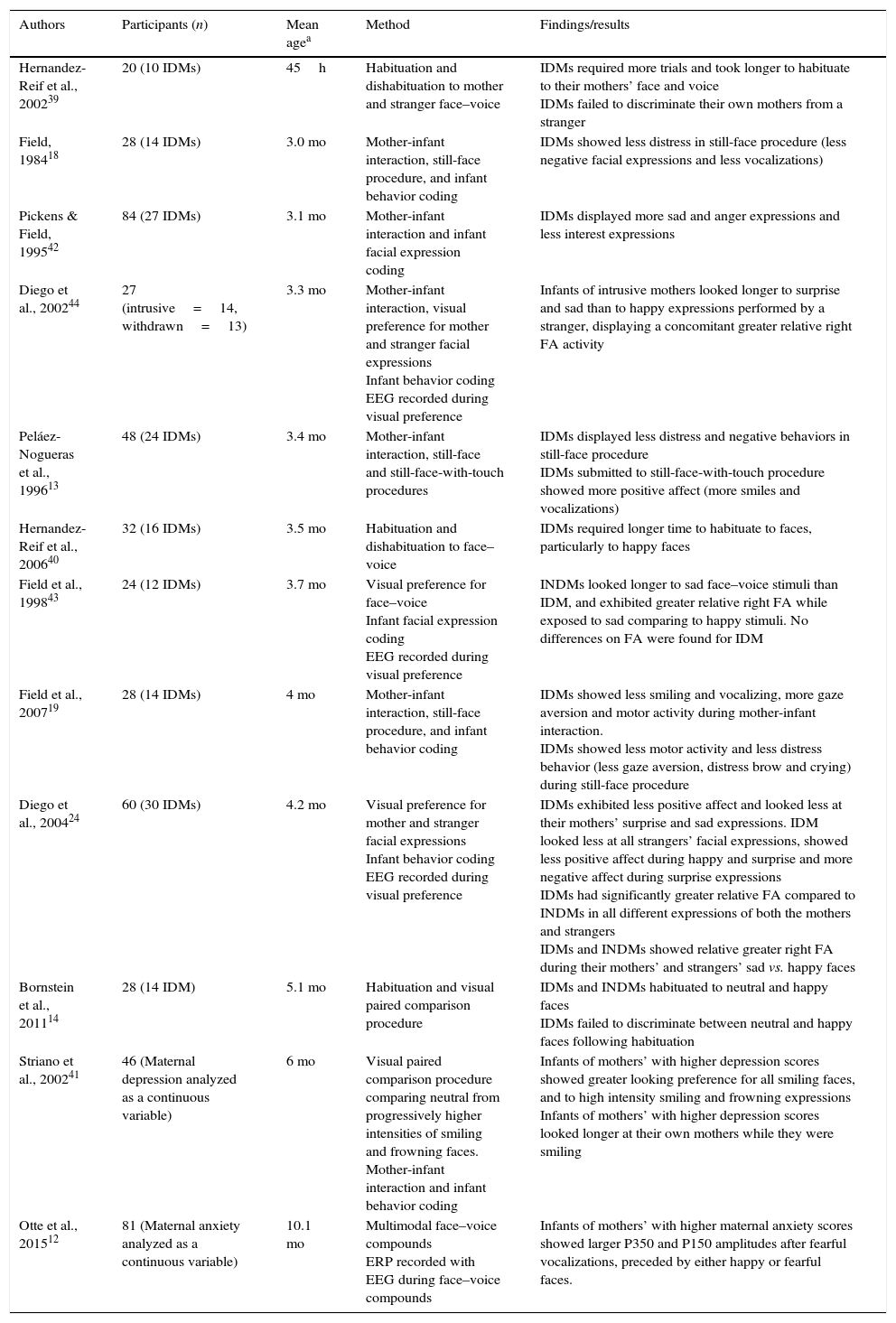
The findings of studies on infants of depressed and anxious mothers are summarized in Table 1.
Studies of behavior and neural correlates on infants of depressed and/or anxious mothers.
| Authors | Participants (n) | Mean agea | Method | Findings/results |
|---|---|---|---|---|
| Hernandez-Reif et al., 200239 | 20 (10 IDMs) | 45h | Habituation and dishabituation to mother and stranger face–voice | IDMs required more trials and took longer to habituate to their mothers’ face and voice IDMs failed to discriminate their own mothers from a stranger |
| Field, 198418 | 28 (14 IDMs) | 3.0 mo | Mother-infant interaction, still-face procedure, and infant behavior coding | IDMs showed less distress in still-face procedure (less negative facial expressions and less vocalizations) |
| Pickens & Field, 199542 | 84 (27 IDMs) | 3.1 mo | Mother-infant interaction and infant facial expression coding | IDMs displayed more sad and anger expressions and less interest expressions |
| Diego et al., 200244 | 27 (intrusive=14, withdrawn=13) | 3.3 mo | Mother-infant interaction, visual preference for mother and stranger facial expressions Infant behavior coding EEG recorded during visual preference | Infants of intrusive mothers looked longer to surprise and sad than to happy expressions performed by a stranger, displaying a concomitant greater relative right FA activity |
| Peláez-Nogueras et al., 199613 | 48 (24 IDMs) | 3.4 mo | Mother-infant interaction, still-face and still-face-with-touch procedures | IDMs displayed less distress and negative behaviors in still-face procedure IDMs submitted to still-face-with-touch procedure showed more positive affect (more smiles and vocalizations) |
| Hernandez-Reif et al., 200640 | 32 (16 IDMs) | 3.5 mo | Habituation and dishabituation to face–voice | IDMs required longer time to habituate to faces, particularly to happy faces |
| Field et al., 199843 | 24 (12 IDMs) | 3.7 mo | Visual preference for face–voice Infant facial expression coding EEG recorded during visual preference | INDMs looked longer to sad face–voice stimuli than IDM, and exhibited greater relative right FA while exposed to sad comparing to happy stimuli. No differences on FA were found for IDM |
| Field et al., 200719 | 28 (14 IDMs) | 4 mo | Mother-infant interaction, still-face procedure, and infant behavior coding | IDMs showed less smiling and vocalizing, more gaze aversion and motor activity during mother-infant interaction. IDMs showed less motor activity and less distress behavior (less gaze aversion, distress brow and crying) during still-face procedure |
| Diego et al., 200424 | 60 (30 IDMs) | 4.2 mo | Visual preference for mother and stranger facial expressions Infant behavior coding EEG recorded during visual preference | IDMs exhibited less positive affect and looked less at their mothers’ surprise and sad expressions. IDM looked less at all strangers’ facial expressions, showed less positive affect during happy and surprise and more negative affect during surprise expressions IDMs had significantly greater relative FA compared to INDMs in all different expressions of both the mothers and strangers IDMs and INDMs showed relative greater right FA during their mothers’ and strangers’ sad vs. happy faces |
| Bornstein et al., 201114 | 28 (14 IDM) | 5.1 mo | Habituation and visual paired comparison procedure | IDMs and INDMs habituated to neutral and happy faces IDMs failed to discriminate between neutral and happy faces following habituation |
| Striano et al., 200241 | 46 (Maternal depression analyzed as a continuous variable) | 6 mo | Visual paired comparison procedure comparing neutral from progressively higher intensities of smiling and frowning faces. Mother-infant interaction and infant behavior coding | Infants of mothers’ with higher depression scores showed greater looking preference for all smiling faces, and to high intensity smiling and frowning expressions Infants of mothers’ with higher depression scores looked longer at their own mothers while they were smiling |
| Otte et al., 201512 | 81 (Maternal anxiety analyzed as a continuous variable) | 10.1 mo | Multimodal face–voice compounds ERP recorded with EEG during face–voice compounds | Infants of mothers’ with higher maternal anxiety scores showed larger P350 and P150 amplitudes after fearful vocalizations, preceded by either happy or fearful faces. |
h, hours; mo, months; IDMs, infants of depressed mothers; INDMs, infants of non-depressed mothers; EEG, electroencephalogram; ERPs, event-related potentials; FA, frontal asymmetry.
In summary, infants of depressed and anxious mothers have increased risk for several detrimental outcomes across the lifespan. They exhibit more difficult temperaments (i.e., highly reactive, fearful, and inhibited) and higher incidence of attentional, emotional, and behavioral problems – such as depression, anxiety, and conduct disorders9,10 – throughout childhood, adolescence, and adulthood.7,9,10
During the first year of life, these infants display a number of atypical behaviors, including less interest in facial expressions, less smiling and vocalizations, more time required to habituate to faces and to face–voice pairs, and failure to discriminate between different emotions.15 Impairments in sensory and perceptual processing as well as reduced attentiveness may underlie such behaviors. Recent neurophysiological studies have begun to shed light on the possible mechanisms linking maternal depression and anxiety to outcomes in infants. EEG studies specifically analyzing facial emotion recognition corroborate findings from the extant literature of a relative right FA asymmetry and its association with depression and negative effects. Comparing to INDMs, IDMs show significantly greater relative right FA across different emotional expressions.24 A recent unique ERP study described a correlation between maternal anxiety and infants’ enhanced ERP components to fearful stimuli, potentially related to an increased bias to threat in these infants.12
Further research is needed to better clarify the potential mechanisms related to infants’ negative outcomes. Expanding the research with current behavior and neurophysiological methods, as well as exploring new tools such as near-infrared spectroscopy, can help detect biologically-based markers that may mediate these associations from the earliest stages of life, months and years prior to adverse clinical outcomes. Fostering clinical and research integration, by incorporating investigation tools in clinical practice or promoting longitudinal studies in risk populations, for example, should facilitate studying individual differences throughout development and enable the potential identification of precocious neural changes in infants associated with the later onset of clinical symptoms. Beyond that, developmental research might eventually contribute to refining screening tools, improving treatment and enabling primary prevention interventions for children at risk.
FundingJuliana A. Porto is supported by CAPES/PDSE at PUCRS and by a research fellowship at the Laboratories of Cognitive Neuroscience, Boston Children's Hospital/Harvard Medical School. Magda L. Nunes is a PQ researcher from CNPq Brazil.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Porto JA, Nunes ML, Nelson CA. Behavioral and neural correlates of emotional development: typically developing infants and infants of depressed and/or anxious mothers. J Pediatr (Rio J). 2016;92(3 Suppl 1):S14–22.